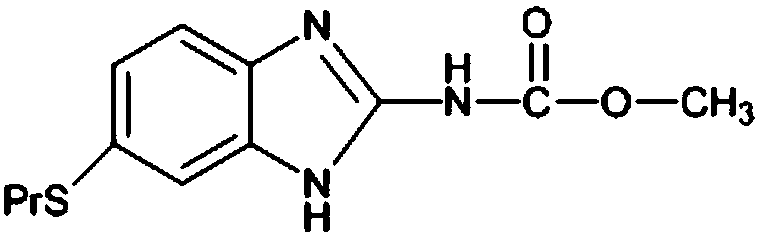
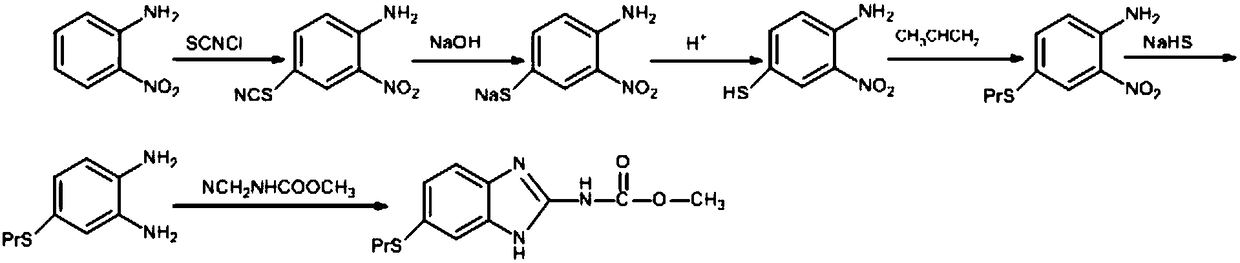
Albendazole synthesis method
A technology of albendazole and a synthesis method, which is applied to the synthesis field of veterinary drugs and pharmaceutical raw material albendazole, can solve the problems of high total yield, multiple yields and the like, and achieves high total yield, clean raw materials and no pollution , the effect of good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment one: One of the preparations of thiocyanate chloride (SCNCl)
[0038] Take 7.60 g (0.10 mol) of ammonium thiocyanate and add it to a four-necked flask with mechanical stirring, add 76.00 ml of methanol, cool and control the temperature to -10°C, and slowly introduce 7.10 g (0.10 mol) of chlorine gas under stirring, and the introduction is complete Continue the insulation reaction for 30 minutes, then filter out the precipitated ammonium chloride, and the filtrate is for subsequent use, wherein the filtrate contains 8.92 grams of thiocyanate chloride (SCNCl), and the yield is 95.37%.
Embodiment 2
[0039] Embodiment two: Chlorothiocyanate (SCNCl) preparation two
[0040] Take 7.60 g (0.10 mol) of ammonium thiocyanate and add it to a four-neck flask with mechanical stirring, add 76.00 ml of methanol, cool and control the temperature to -10°C, and slowly introduce 7.81 g (0.11 mol) of chlorine gas under stirring, and the introduction is complete Continue the insulation reaction for 30 minutes, then filter out the precipitated ammonium chloride, and the filtrate is for subsequent use, wherein the filtrate contains 9.26 grams of chlorothiocyanate (SCNCl), and the yield is 99.02%.
Embodiment 3
[0041] Embodiment three: Thiocyanine Chloride (SCNCl) Preparation Three
[0042]Take 7.60 g (0.10 mol) of ammonium thiocyanate and add it to a four-neck flask with mechanical stirring, add 76.00 ml of methanol, cool and control the temperature to -10°C, and slowly introduce 9.23 g (0.13 mol) of chlorine gas under stirring, and the introduction is complete Continue the insulation reaction for 30 minutes, then filter out the precipitated ammonium chloride, and the filtrate is for subsequent use, wherein the filtrate contains 9.24 grams of thiocyanate chloride (SCNCl), and the yield is 98.78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com