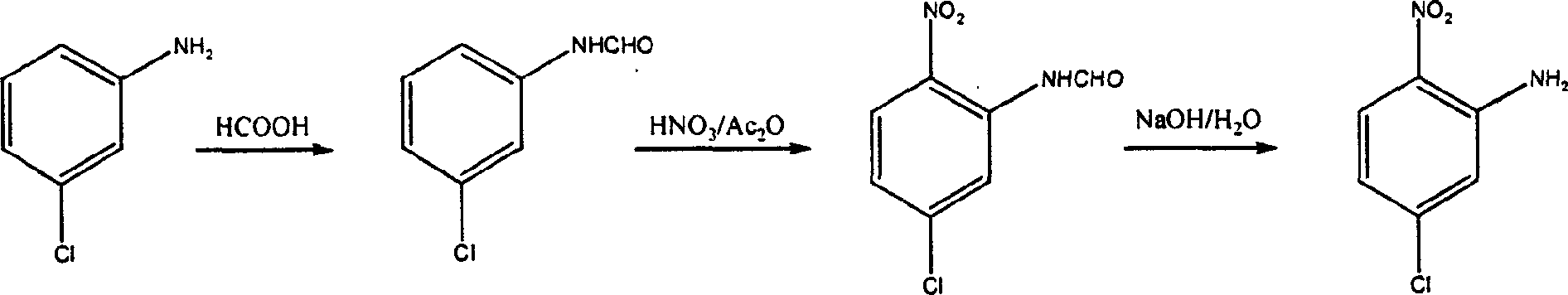
Chemical synthesizing method for 5-chlorine-2-nitroaniline
A technology for the synthesis of nitroaniline, which is applied in the fields of chemical instruments and methods, preparation of amino compounds, organic chemistry, etc., can solve the problems of high cost and long reaction time, and achieve the advantages of convenient processing, high selectivity and shortened reaction time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] 3-Chloroformanilide: In a 250ml three-necked flask equipped with a water separator, add 51.5g (0.41mol) 3-chloroaniline and 150ml toluene, stir to dissolve, then slowly add 23.5g (0.45mol) 88% formic acid , heat up and reflux for 1h, cool to 65-70°C, add 6.4g (0.12mol) 88% formic acid, heat up and reflux again for 1h, cool to room temperature, remove toluene and excess formic acid on a rotary evaporator, cool to obtain white Solid 3-chloroformanilide 61.7g, yield 97%, m.p.51~53℃.
Embodiment 2
[0018] 3-Chloroformanilide: except that benzene was used instead of toluene, other operating conditions were the same as in Example 1 to obtain 53.8 g of 3-chloroformanilide as a white solid with a yield of 84%, m.p.50-53°C.
Embodiment 3
[0020] 3-Chloroformanilide: except that n-butyl ether was used instead of toluene, other operating conditions were the same as in Example 1 to obtain 58.2 g of 3-chloroformanilide as a white solid, yield 91%, m.p.51-53°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com