New process synthesis method for 2-sulfydryl-5-methoxybenzimidazole
A technology for the synthesis of methoxybenzimidazole and its synthesis method, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of unfavorable green and environmental protection industrial production, operators and environmental hazards, poor operation safety, etc., and achieve the optimization and improvement of the process synthesis route method, Effect of improving drug safety and reducing preparation cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097]
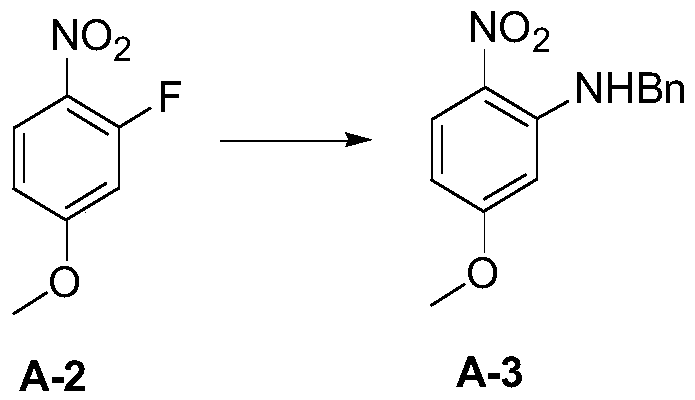
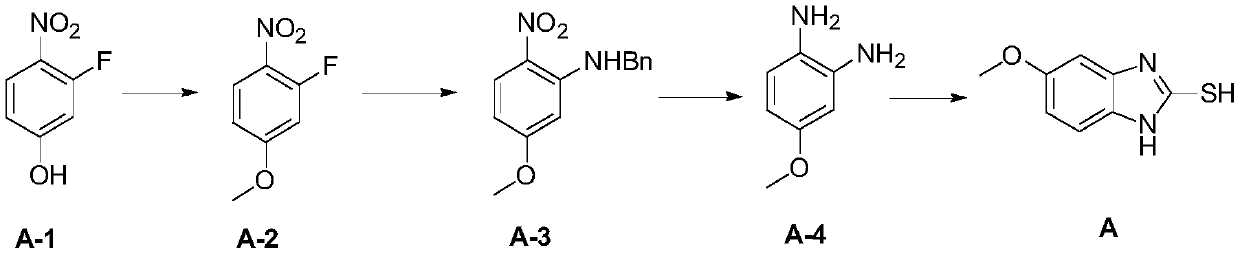
[0098] 1) Put 8.8 g of potassium carbonate and 150 mL of DMF into the reaction flask, add 5.0 g of compound A-1 and 13.5 g of methyl iodide, and heat to 40° C. and stir for 18 hours. The reaction liquid was cooled, filtered with suction, washed with water, dried, and concentrated to obtain compound A-2 (4.8 g, yield 89%);
[0099] 2) Add 0.5 g of compound A-2 and 5.2 mL of DMF to the reaction flask, add 0.8 g of potassium carbonate and 0.3 g of benzylamine in turn, the reaction solution is heated to 80°C and stirred for 4 hours, filtered, concentrated under reduced pressure to remove the solvent, washed with water, and dried Obtain compound A-3 (0.73 g, yield 97%);
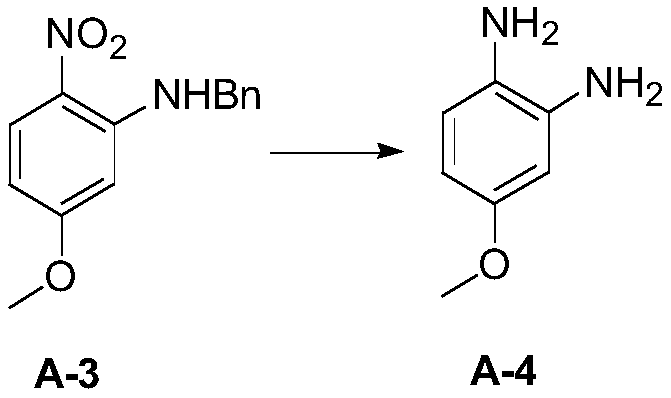
[0100] 3) Add 0.3 g of compound A-3 and 10 mL of methanol into the reaction flask, add 0.06 g of 10% palladium on carbon, replace the hydrogen with the reaction solution at 30°C, stir for 20 hours, filter, and concentrate under reduced pressure to remove the solvent to obtain compound A-4 (0.16g , The yield i...
Embodiment 2
[0103]
[0104] 1) The compound A-2 was obtained by the operation similar to step 1 of Example 1;
[0105] 2) Add 0.5 g of compound A-2 and 3 mL of DMF to the reaction flask, add 0.4 g of cesium carbonate and 0.6 g of benzylamine in turn, the reaction solution is heated to 100°C and stirred for 2 hours, filtered, concentrated under reduced pressure to remove the solvent, washed with water, and dried to obtain Compound A-3 (0.7g, yield 95%);
[0106] 3) Add 0.3g of compound A-3 and 9mL of methanol into the reaction flask, add 0.03g of 5% palladium on carbon, replace the hydrogen with the reaction solution at 60°C, stir for 12h, filter, concentrate under reduced pressure to remove the solvent to obtain compound A-4 (0.14g , The yield is 96%);
[0107] 4) Add 0.1 g of compound A-4, 0.04 g of potassium hydroxide, 0.07 g of carbon disulfide and 10 mL of ethanol into the reaction flask. The reaction solution was heated to 60°C and stirred for 14 hours, a small amount of activated carbon w...
Embodiment 3
[0109]
[0110] 1) The compound A-2 was obtained by the operation similar to step 1 of Example 1;
[0111] 2) Add 0.5 g of compound A-2 and 10 mL of DMF to the reaction flask, add 1.6 g of potassium hydroxide and 0.45 g of benzylamine in turn, the reaction solution is heated to 120°C and stirred for 8 hours, filtered, concentrated under reduced pressure to remove the solvent, washed with water, and dried Obtain compound A-3 (0.75g, yield 98%);
[0112] 3) Add 0.3g of compound A-3 and 12mL of methanol into the reaction flask, add 0.09g of zinc powder, the reaction solution replaces hydrogen at 20°C and stirred for 48h, filtered, and concentrated under reduced pressure to remove the solvent to obtain compound A-4 (0.12g, yield 95%);
[0113] 4) Add 0.1 g of compound A-4, 0.075 g of potassium hydroxide, 0.07 g of carbon disulfide and 30 mL of ethanol into the reaction flask, the reaction solution was heated to 70°C and stirred for 4 hours, a small amount of activated carbon was added, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com