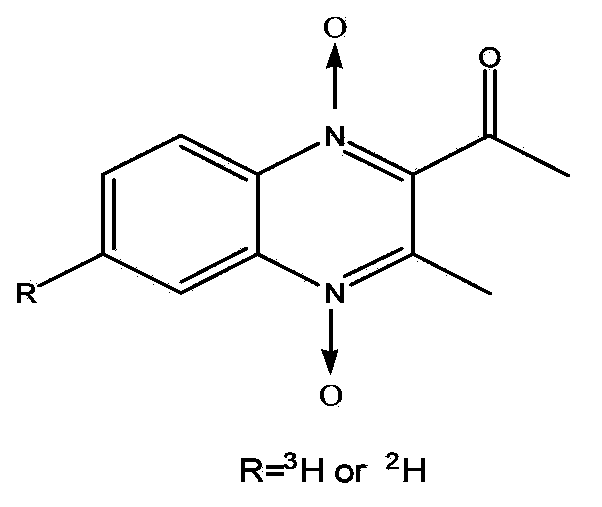
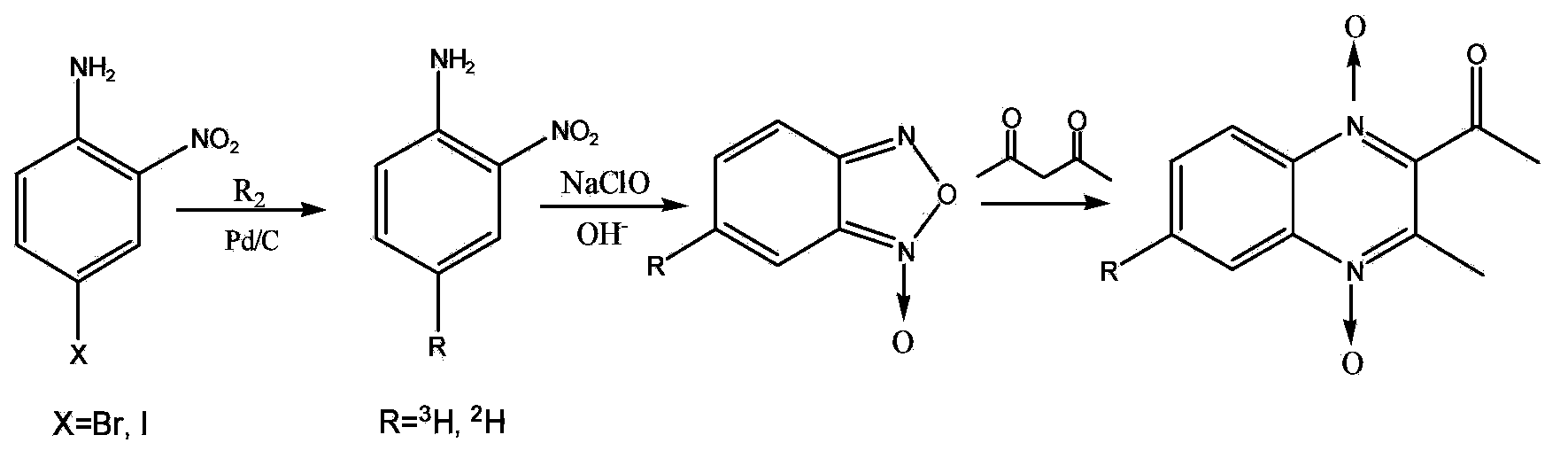
Preparation method for tritium or deuterium labeled mequindox
A technology of acemequine and deuterium labeling, applied in the direction of organic chemistry, etc., can solve the problems of not meeting the requirements of drug tracer research, no public reports or patents, and inability to explain drug metabolism characteristics, etc., so that the reaction conditions are easy to grasp, The effect of high specific activity and suitable synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Synthesis of Tritiated Methoquine
[0043] Take 20 mg of 4-bromo-2-nitroaniline, 5 mg of palladium / carbon (Pd / C) catalyst with a mass ratio of 10%, 4 mg of sodium hydroxide, and 1 ml of ethanol are added to a 25 ml reaction bottle in turn, and sealed to the glass tritiation reaction On the device, vacuumize, pass tritium (or deuterium) gas, the pressure in the reaction bottle reaches 200mmHg, the reaction temperature is 10°C, stir the reaction for 45min, stop stirring, freeze with liquid nitrogen, recover the tritium gas, let it melt, and collect the catalyst by suction filtration. Preparative chromatographic separation gives [ 3 H]-o-nitroaniline. Yield 67.2%.
[0044] Put into the 3ml reaction bottle [ 3 H]-o-nitroaniline 15 mg, isopropanol 100 μl, sodium hydroxide 6 mg. Slowly drop 100 μl of sodium hypochlorite solution with an effective chlorine molar ratio of 5% under stirring, react at 10°C for 2 hours, let stand for 1 hour, filter with suction, wash...
Embodiment 2
[0046] Example 2 Synthesis of Tritiated Methoquine
[0047] Take 25 mg of 4-iodo-2-nitroaniline, 5 mg of Pd / C catalyst with a mass ratio of 10%, 4 mg of sodium hydroxide, and 1 ml of ethanol are sequentially added to a 25 ml reaction bottle, sealed and connected to the tritiation reaction device, and vacuumized. Pass tritium (or deuterium) gas, the pressure in the reaction bottle reaches 200mmHg, the reaction temperature is 10°C, stir the reaction for 5 minutes, stop the stirring, freeze with liquid nitrogen, recover the tritium gas, let it melt, and collect the catalyst by suction filtration. Preparative chromatographic separation gives [ 3 H]-o-nitroaniline. Yield 68.4%.
[0048] Put into the 3ml reaction bottle [ 3 H]-o-nitroaniline 15 mg, isopropanol 100 μl, sodium hydroxide 6 mg. Slowly drop 100 μl of sodium hypochlorite solution with an effective chlorine molar ratio of 5% under stirring, react at 10°C for 2 hours, let stand for 1 hour, filter with suction, wash the ...
Embodiment 3
[0050] Example 3 Synthesis of Tritiated Methoquine
[0051] The operating method of this embodiment is the same as that of Example 1 respectively, the debromination reaction catalyst is respectively Raney nickel, mass ratio is 10% palladium / carbon (Pd / C) catalyst, palladium chloride, and the acid acceptor is respectively sodium acetate , Potassium carbonate, the results are basically the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com