Preparation method of fenbendazole
A technology for the preparation of fenbendazole, which is applied in the field of preparation of fenbendazole, can solve the problems of high production cost and achieve the effects of reduced production cost, sufficient reaction and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
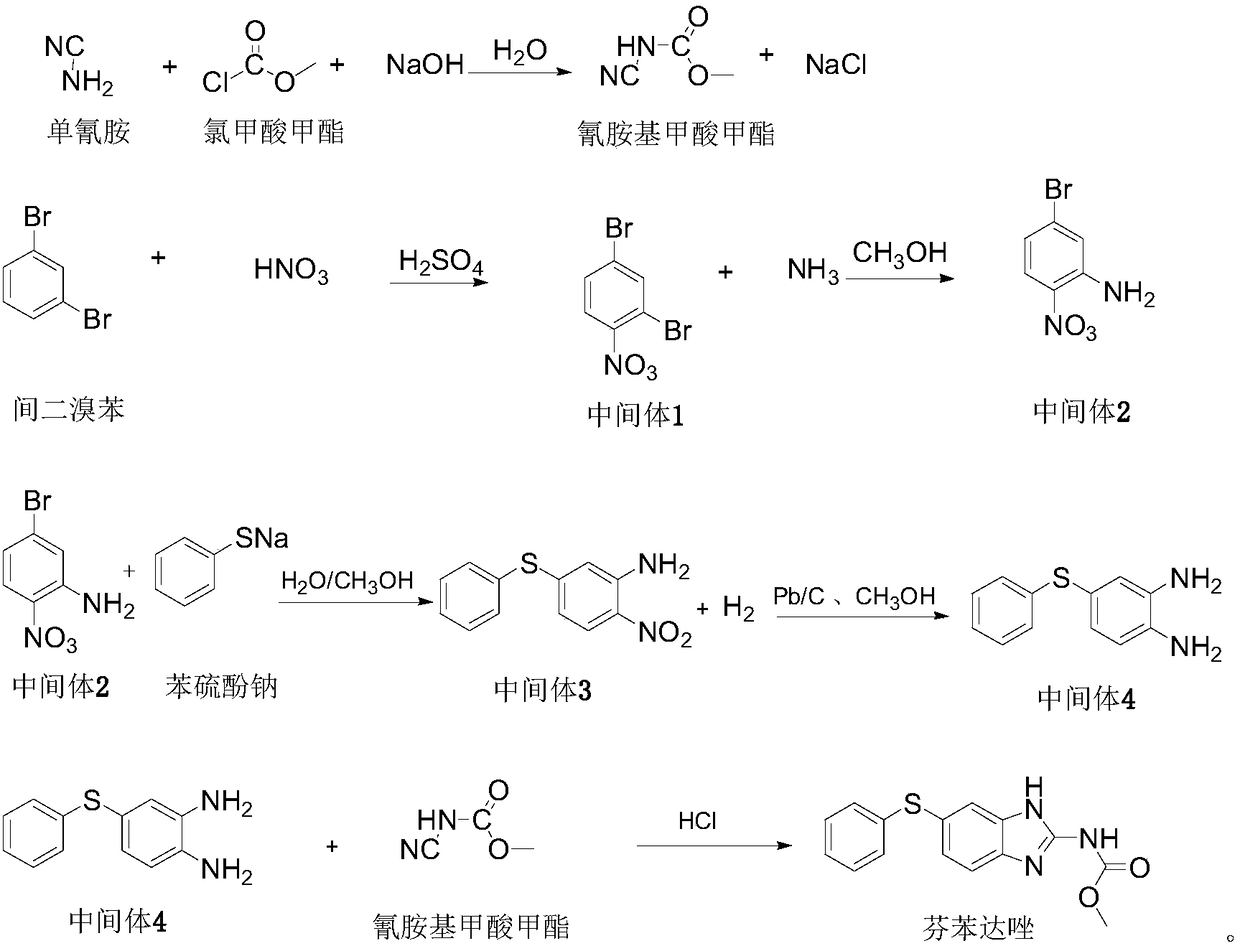
[0016] (1) Preparation of methyl cyanamide formate aqueous solution
[0017] 44.0g of cyanamide aqueous solution is cooled to 0-5°C, and 10.93g of methyl chloroformate and 16.03g of sodium hydroxide solution (30%) are added dropwise under stirring, and the temperature of the dropping process is controlled at 0-5°C. The materials drop off at the same time or the methyl chloroformate drops slightly earlier than the sodium hydroxide solution. After both materials were added dropwise, stir at 0-5°C for 2 hours to obtain 70.91 g of an aqueous solution of methyl cyanamide with a concentration of 14.0%, and store it at a low temperature around 0°C for future use.
[0018] (2) Intermediate 1 (2,4-dibromonitrobenzene)
[0019] Dissolve 24.5g of m-dibromobenzene in 49.0g of concentrated sulfuric acid (98%) at room temperature, cool to 0-5°C, add 9.80g of nitric acid (68%) dropwise, and control the temperature at 0-5°C during the dropping process. After the addition was complete, stir ...
Embodiment 2
[0029] (1) Preparation of methyl cyanamide formate aqueous solution
[0030] 220.0g of cyanamide aqueous solution was cooled to 0-5°C, and 54.65g of methyl chloroformate and 80.15g of sodium hydroxide solution (30%) were added dropwise under stirring, and the temperature of the dropping process was controlled at 0-5°C. The materials drop off at the same time or the methyl chloroformate drops slightly earlier than the sodium hydroxide solution. After the addition of the two materials was completed, stir at 0-5°C for 2 hours to obtain 355.0 g of an aqueous solution of methyl cyanamide with a concentration of 14.0%, and store it at a low temperature of about 0°C for future use.
[0031] (2) Intermediate 1 (2,4-dibromonitrobenzene)
[0032] Dissolve 122.5g m-dibromobenzene in 245.0g concentrated sulfuric acid (98%) at room temperature, cool to 0-5°C, add 49.0g nitric acid (68%) dropwise, control the temperature at 0-5°C during the dropwise addition, After the addition was comple...
Embodiment 3
[0042] (1) Preparation of methyl cyanamide formate aqueous solution
[0043] 440.0g of cyanamide aqueous solution is cooled to 0-5°C, and 109.3g of methyl chloroformate and 160.3g of sodium hydroxide solution (30%) are added dropwise under stirring, and the temperature of the dropping process is controlled at 0-5°C. The materials drop off at the same time or the methyl chloroformate drops slightly earlier than the sodium hydroxide solution. After both materials were added dropwise, stir at 0-5°C for 2 hours to obtain 708.0 g of methyl cyanamide aqueous solution with a concentration of 14.0%, and store it at a low temperature of about 0°C for future use.
[0044] (2) Intermediate 1 (2,4-dibromonitrobenzene)
[0045] Dissolve 245.0g m-dibromobenzene in 490.0g concentrated sulfuric acid (98%) at room temperature, cool to 0-5°C, add 98.0g nitric acid (68%) dropwise, control the temperature at 0-5°C during the dropping process, and drop After the addition was complete, stir at 5-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com