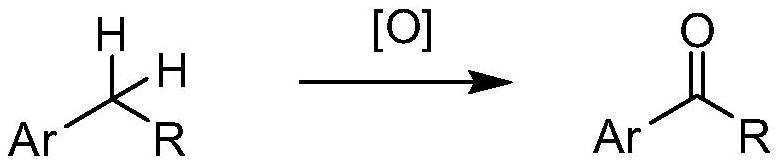A method for direct oxidation of benzylic c-h bond into ketone or acid
A benzylic and direct technology, applied in the field of chemical catalytic oxidation, can solve problems such as environmental pollution, high equipment requirements, and impact on healthy life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
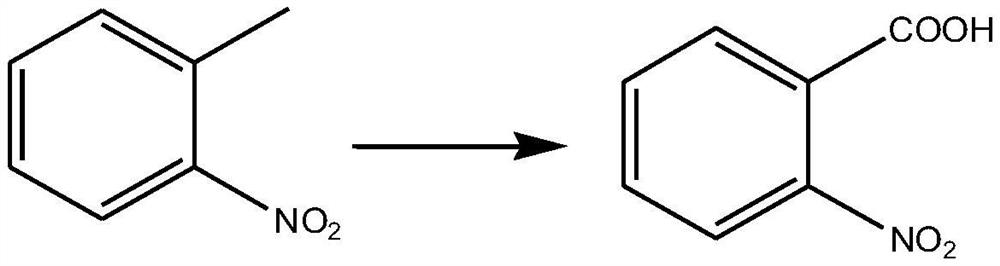
Embodiment 1
[0027] Preparation of o-nitrobenzoic acid:
[0028]
[0029] Take 0.2mol of o-nitrotoluene, 200ml of dichloromethane, 20ml of water, 0.02mol of sodium bromide, 0.001mol of tetramethylpiperidine nitrogen oxide and mix well, raise the temperature to 40°C, and add a total of 0.24mol of Trichloroisocyanuric acid, keep stirring for 12 hours, add 50ml methanol to quench the reaction, filter the filtrate, extract 3 times with dichloromethane, each time with 100ml dichloromethane, combine the organic phases, wash with 400ml saturated aqueous sodium chloride solution , dried over anhydrous sodium sulfate, distilled off the organic solvent under reduced pressure, and recrystallized with a mixed solvent of ethyl acetate and petroleum ether to obtain o-nitrobenzoic acid with a yield of 94.2%.
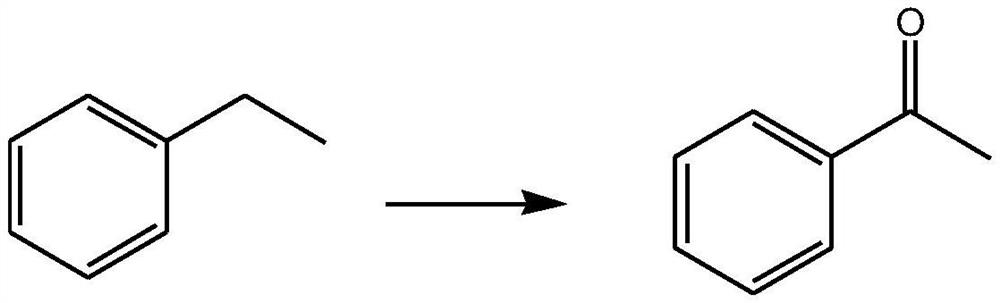
Embodiment 2
[0031] To prepare acetophenone:
[0032]
[0033] Take 0.2mol ethylbenzene, 200ml dichloromethane, 20ml water, 0.02mol sodium bromide, 0.001mol tetramethylpiperidine nitrogen oxide and mix well, raise the temperature to 40°C, add a total of 0.24mol trichloride in 4 times Isocyanuric acid, keep stirring for 12 hours, add 50ml of methanol to quench the reaction, filter the filtrate, extract 3 times with dichloromethane, each time with 100ml of dichloromethane, combine the organic phases, wash with 400ml saturated aqueous sodium chloride solution, no Dry over sodium sulfate, distill off the organic solvent under reduced pressure, and recrystallize with a mixed solvent of ethyl acetate and petroleum ether to obtain acetophenone with a yield of 96.2%.
Embodiment 3
[0035] Preparation of phthalic acid:
[0036]
[0037] Take 0.2mol of o-xylene, 200ml of dichloromethane, 20ml of water, 0.04mol of sodium bromide, and 0.002mol of tetramethylpiperidine nitrogen oxide, mix them evenly, raise the temperature to 40°C, and add a total of 0.48mol of three Chloroisocyanuric acid, keep stirring for 12h, add 50ml methanol to quench the reaction, filter the filtrate, extract 3 times with dichloromethane, each time with 100ml dichloromethane, combine the organic phases, wash with 400ml saturated aqueous sodium chloride solution, Dry over anhydrous sodium sulfate, distill off the organic solvent under reduced pressure, and recrystallize with a mixed solvent of ethyl acetate and petroleum ether to obtain phthalic acid with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com