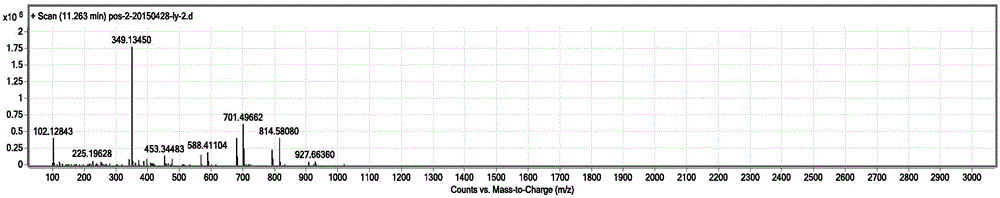
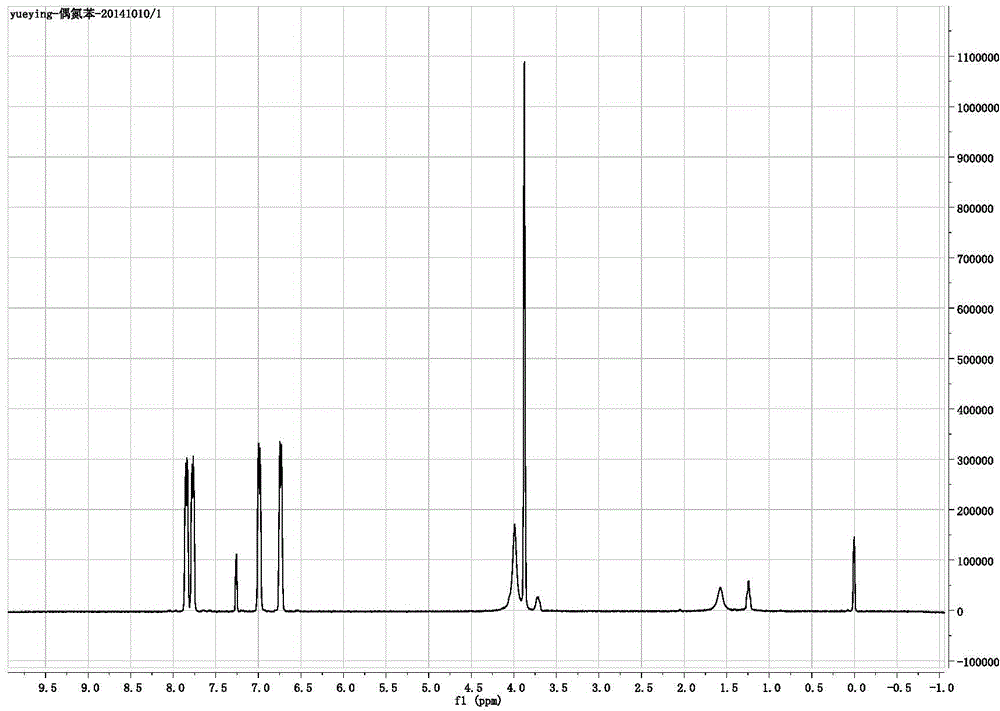
Novel photochromic azobenzene compound and synthesis method thereof
A synthetic method and photochromic technology, applied in the direction of organic chemistry, can solve the problems of anhydrous and oxygen-free solvent treatment, expensive catalysts, etc., achieve significant economic and environmental benefits, simple post-treatment of products, and high conversion rate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] A preparation method of a novel azobenzene compound N-[4-[2-(4-methoxyphenyl)diazenyl]phenyl]-2-nitroaniline (compound 4), comprising the following steps :
[0046] (1) In the 250mL three-necked flask, add 6.15g compound 1, 12.9mL mass fraction is 36% hydrochloric acid, 100mL deionized water, stir and make it dissolve completely, obtain homogeneous mixed solution, add to described mixed solution under stirring condition 38 mL of NaNO with a mass fraction of 10% was added dropwise to 2 Solution, stirred and reacted for 1 h at 0-5°C to obtain a diazotization reaction solution, namely an aqueous solution of compound 2;
[0047] (2) add 4.56g aniline in the 500mL three-necked flask, 4.3mL mass fraction is the hydrochloric acid of 36%, 100mL deionized water, stir and make it fully dissolve, form the coupling liquid of aniline and hydrochloric acid; Step (1) gained The diazotization reaction solution is slowly added dropwise to the coupling solution, the temperature of the ...
Embodiment 2
[0050] A preparation method of a novel azobenzene compound N-[4-[2-(4-methoxyphenyl)diazenyl]phenyl]-2-nitroaniline (compound 4), comprising the following steps :
[0051] Step (1) and step (2) of the present embodiment adopt the same steps as step (1) and step (2) in embodiment 1;
[0052] Step (3): Add 0.1g of compound 3, 0.104g of 2-nitrochlorobenzene, and 0.064g of KF / Al to a 50mL single-necked round bottom flask. 2 o 3 , N,N-Dimethylformamide 5mL, reflux at 130°C for 3h, after the reaction is complete, remove the catalyst by filtration, transfer the filtrate to a separatory funnel, add 20mL ethyl acetate and 20mL saturated saline to remove the solvent N,N-di Methylformamide, so repeatedly washed 2-3 times, separated the organic layer, and distilled off the solvent under reduced pressure to obtain the crude product. The crude product was obtained, and the crude product was separated by column chromatography. The mixed solution of ethyl acetate and sherwood oil is eluent...
Embodiment 3
[0054] A preparation method of a novel azobenzene compound N-[4-[2-(4-methoxyphenyl)diazenyl]phenyl]-2-nitroaniline (compound 4), comprising the following steps :
[0055] Step (1) and step (2) of the present embodiment adopt the same steps as step (1) and step (2) in embodiment 1;
[0056] Step (3): Add 0.1g of compound 3, 0.138g of 2-nitrochlorobenzene, and 0.064g of KF / Al to a 50mL single-necked round bottom flask. 2 o 3 , N,N-Dimethylformamide 5mL, reflux at 130°C for 3h, after the reaction is complete, remove the catalyst by filtration, transfer the filtrate to a separatory funnel, add 20mL ethyl acetate and 20mL saturated saline to remove the solvent N,N-di Methylformamide, so repeatedly washed 2-3 times, separated the organic layer, and distilled off the solvent under reduced pressure to obtain the crude product, which was separated by column chromatography, and 300 mL of ethyl acetate with a volume ratio of 1:15 The mixed solution with sherwood oil is eluent elution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com