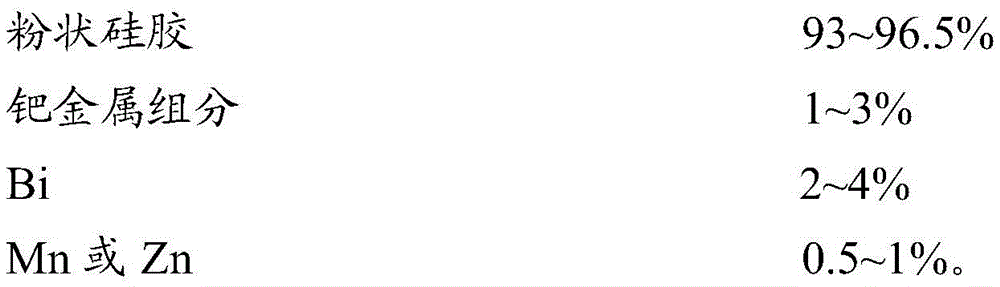Patents
Literature
31results about How to "Many side effects" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Method for synthesizing triallyl phosphate
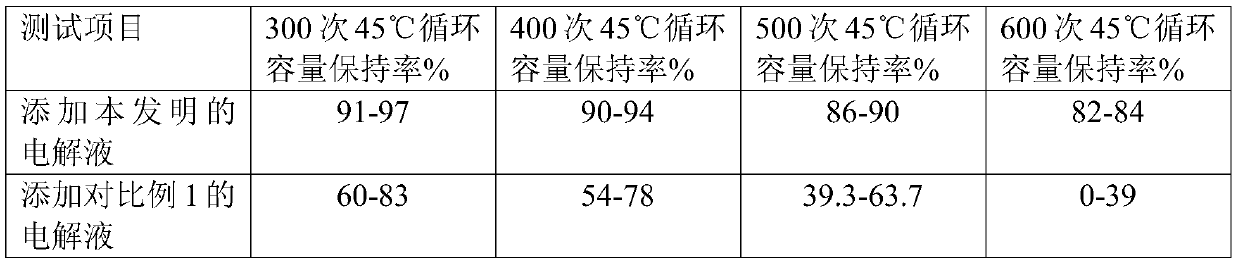
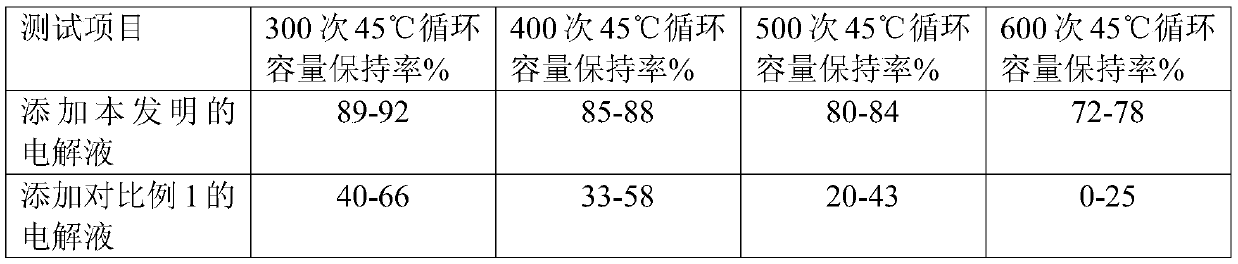
ActiveCN106957332ALow purityLow yieldGroup 5/15 element organic compoundsPhosphateReaction temperature
The invention provides a method for synthesizing triallyl phosphate, and belongs to the technical field of compound synthesis. The method comprises the following steps: adding allyl alcohol serving as a raw material and an acid-binding agent into a solvent under protection of nitrogen; then, adding phosphorus oxychloride to react; pumping and filtering the reacting liquid; washing, drying, decoloring and concentrating to obtain triallyl phosphate; controlling the reacting temperature to be less than negative 30 DEG C when the phosphorus oxychloride is dropwise added for 3.4-7.5 hours; and naturally heating to -2-zero DEG C, and stopping reaction. The synthesizing method is simple and easy to operate, and the prepared triallyl phosphate has high yield and high purity.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
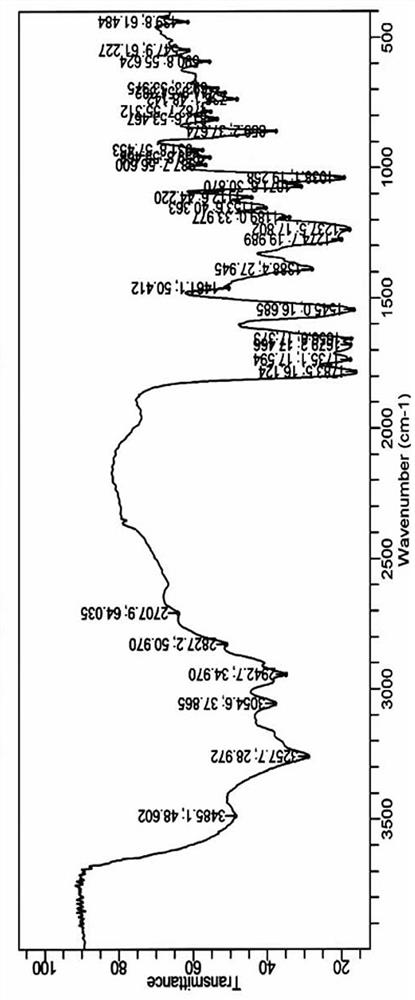
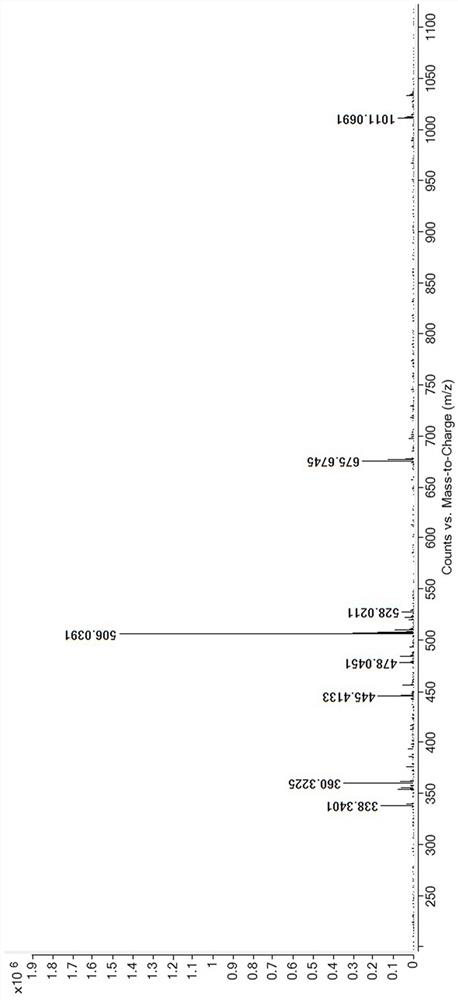
Method for producing 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide
A method for producing 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide comprises an addition reaction, an oxidation reaction and a purifying process. The method concretely comprises the following steps: 1, carrying out the addition reaction: carrying out addition reaction on thionyl chloride and 1,2-pentanediol as reaction raw materials; 2, carrying out the oxidation reaction: adding a sodium hypochlorite and catalyst mixed solution, carrying out the oxidation reaction to obtain a water phase and organic phase co-existence reaction solution, standing the water phase and organic phase co-existence reaction solution for layering, and separating the obtained water phase to obtain the organic phase which is crude 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide; and 3, purifying: carrying out molecular distillation to obtain 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide. The purity of the prepared 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide can be greater than 99.5%, the water content is not greater than 100PPM, the acid value is not greater than 100PPM, and 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide can be added to a battery in order to improve the performances of the battery and prolong the life of the battery.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
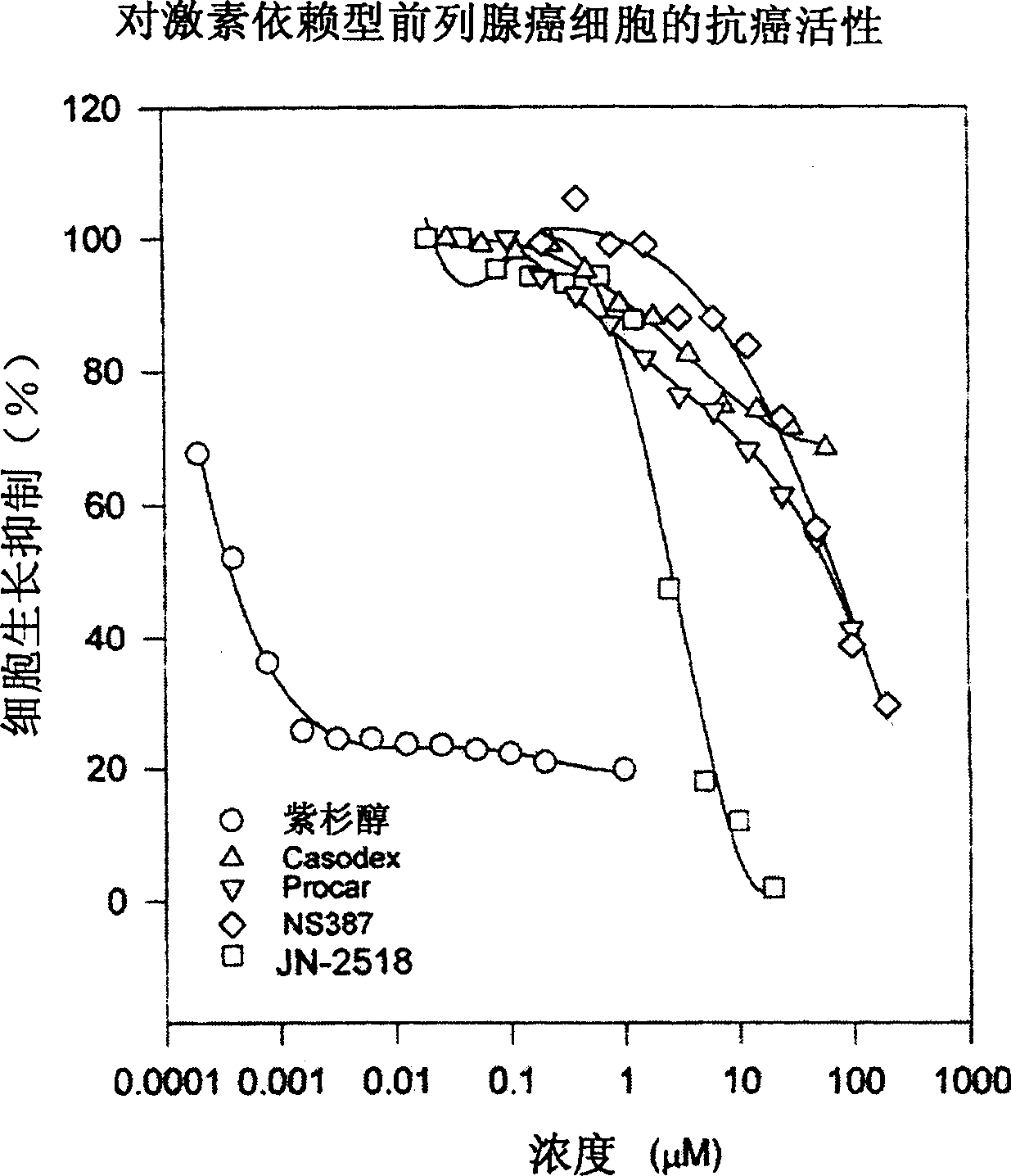
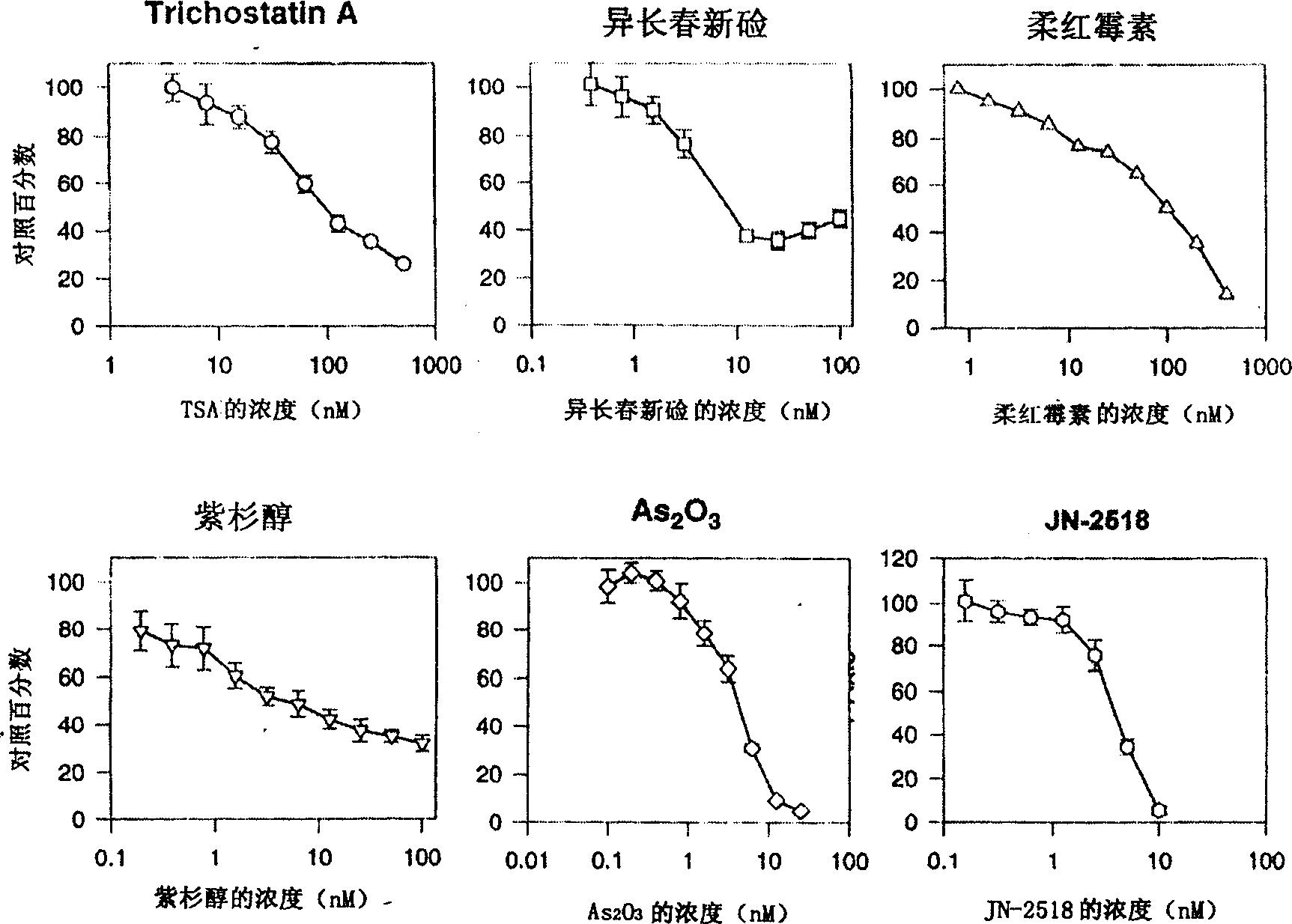
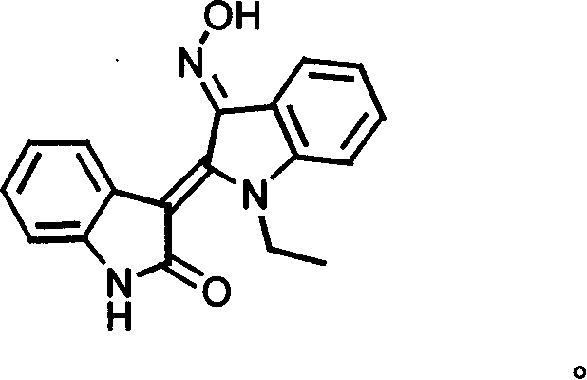
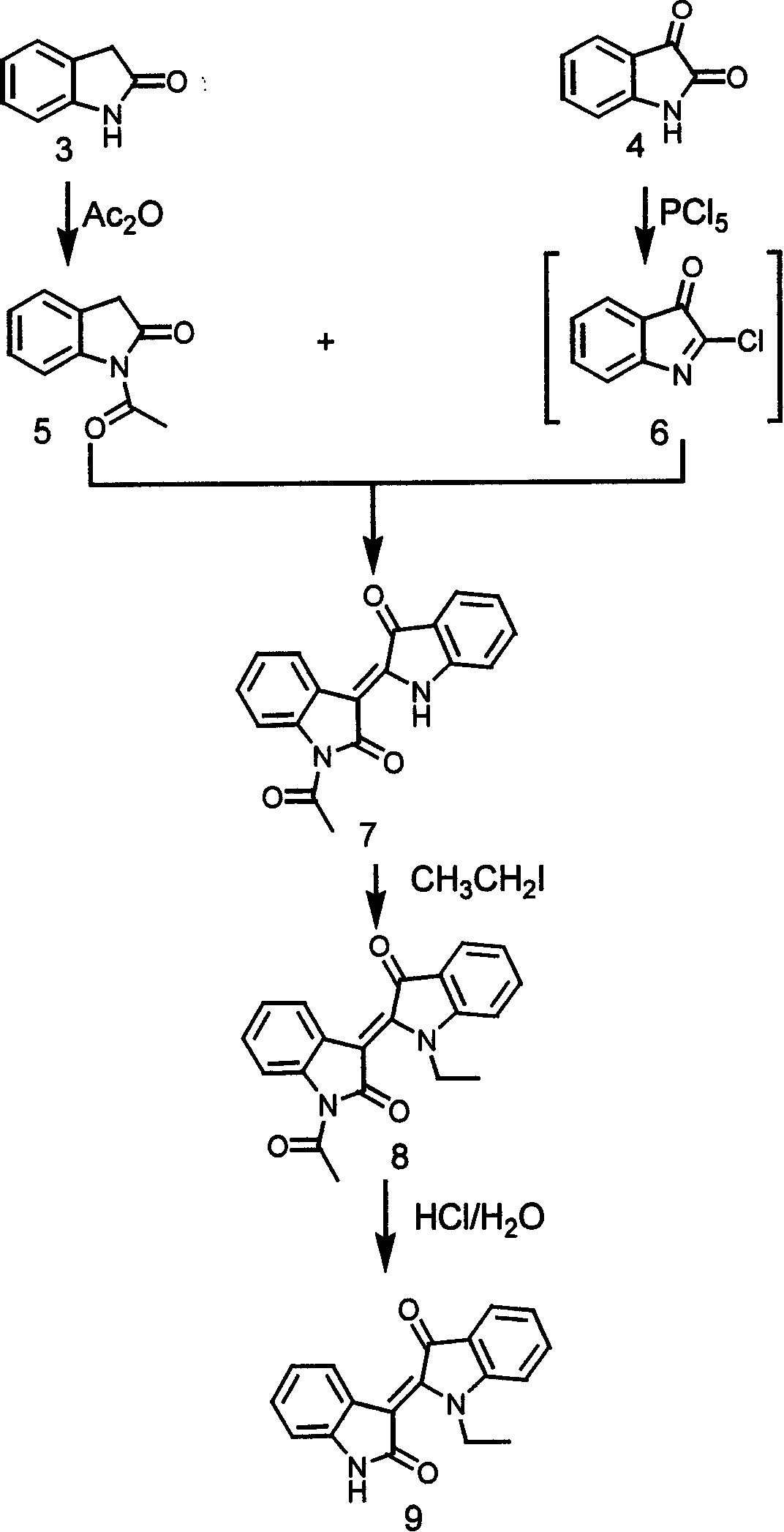
Specific indole compound and its preparation and use in treating and preventing cancers
InactiveCN1424312APrevent proliferationInduce apoptosisOrganic active ingredientsNervous disorderOrganic synthesisMedicine
An organic heterocycle compound series JN-2158 is prepared by organic synthetic reaction. It can be used in medicine field to treat more diseases of people and animal, such as cancers, senitile dementia, psoriasis, cardiovascular diseas, glomerulonephritis, etc and prevent and cure AIDS.
Owner:JC (WUXI) CO INC
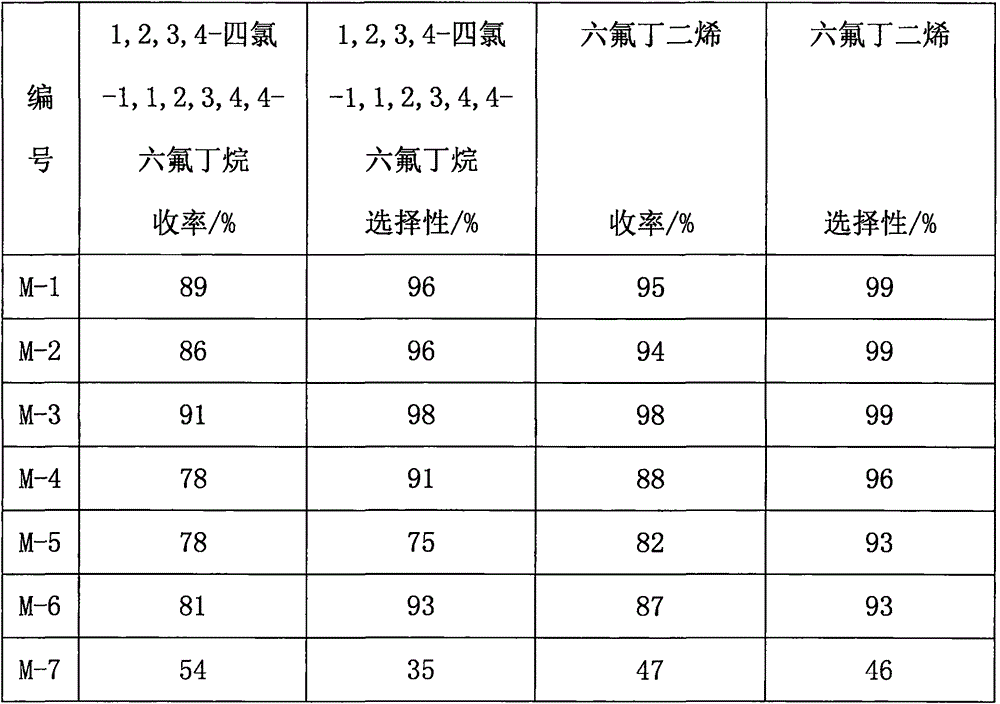
Method for preparing hexafluorobutadiene from iodine and chlorine
InactiveCN106336342AExtended reaction timeMany side effectsPreparation by dehalogenationPreparation by halogen additionSolventTrifluoroethane
The invention relates to a method for preparing hexafluorobutadiene from iodine and chlorine. The method comprises the following steps: preparing a metal coordinated ionic liquid solvent, and reacting iodine with chlorine to prepare iodine monochloride; reacting iodine monochloride with chlorotrifluoroethylene in the presence of the metal coordinated ionic liquid solvent to prepare 1,2-dichloro-2-iodo-1,1,2-trifluoroethane; carrying out a reaction on the 1,2-dichloro-2-iodo-1,1,2-trifluoroethane in the presence of the metal coordinated ionic liquid solvent under the catalysis of zinc powder to obtain 1,2,3,4-tetrachloro-1,1,2,3,4,4-hexafluorobutane; and reacting the 1,2,3,4-tetrachloro-1,1,2,3,4,4-hexafluorobutane with zinc powder in the presence of the metal coordinated ionic liquid solvent to generate hexafluorobutadiene.
Owner:ZHEJIANG BRITECH CO LTD
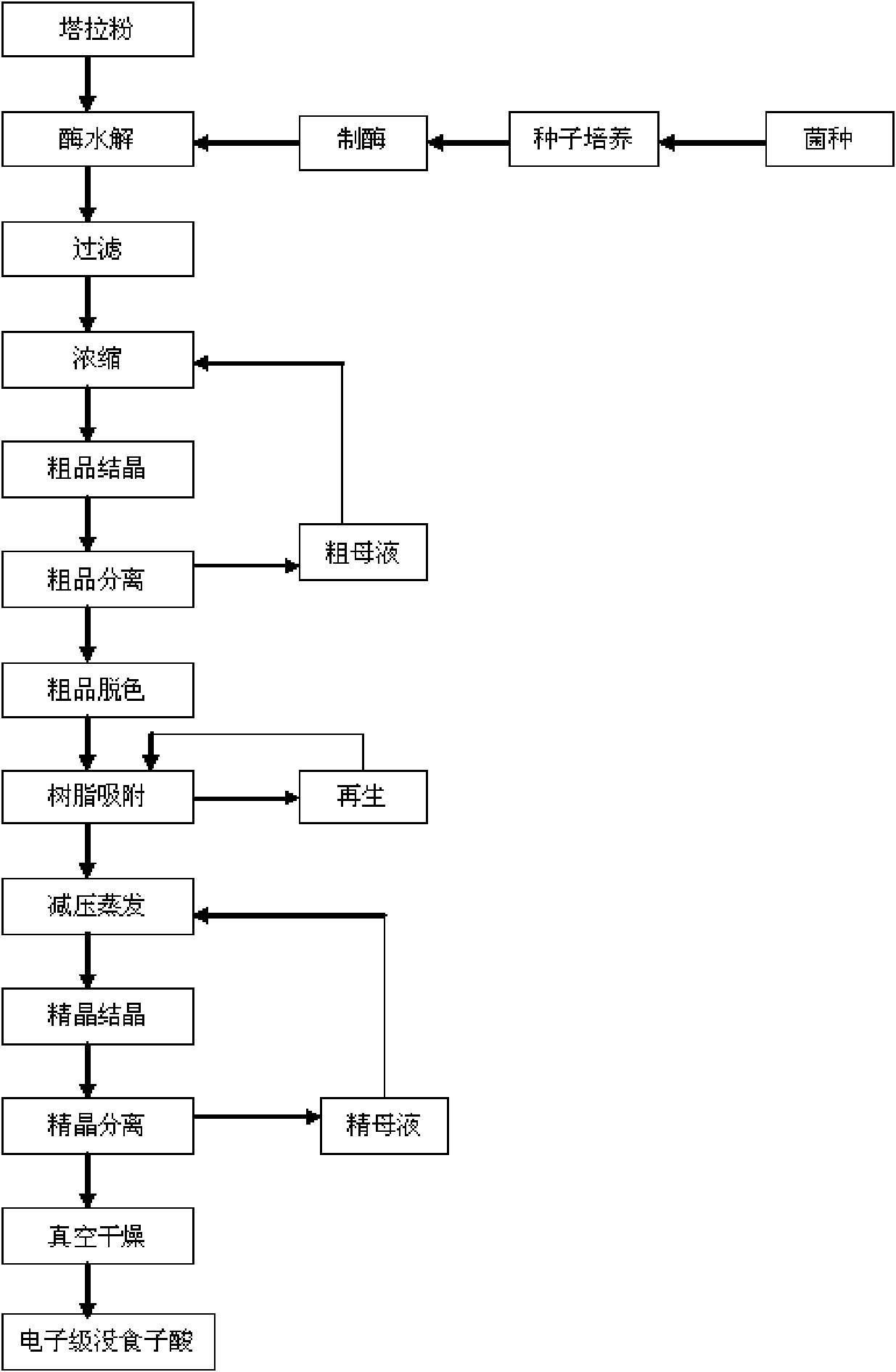
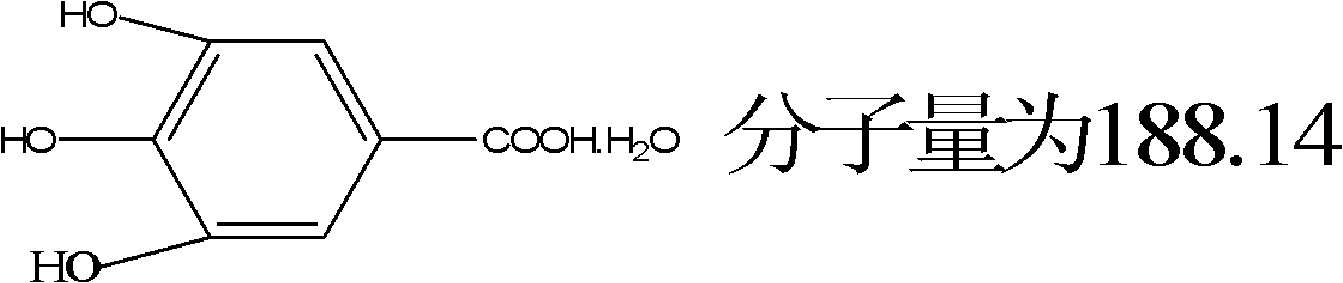
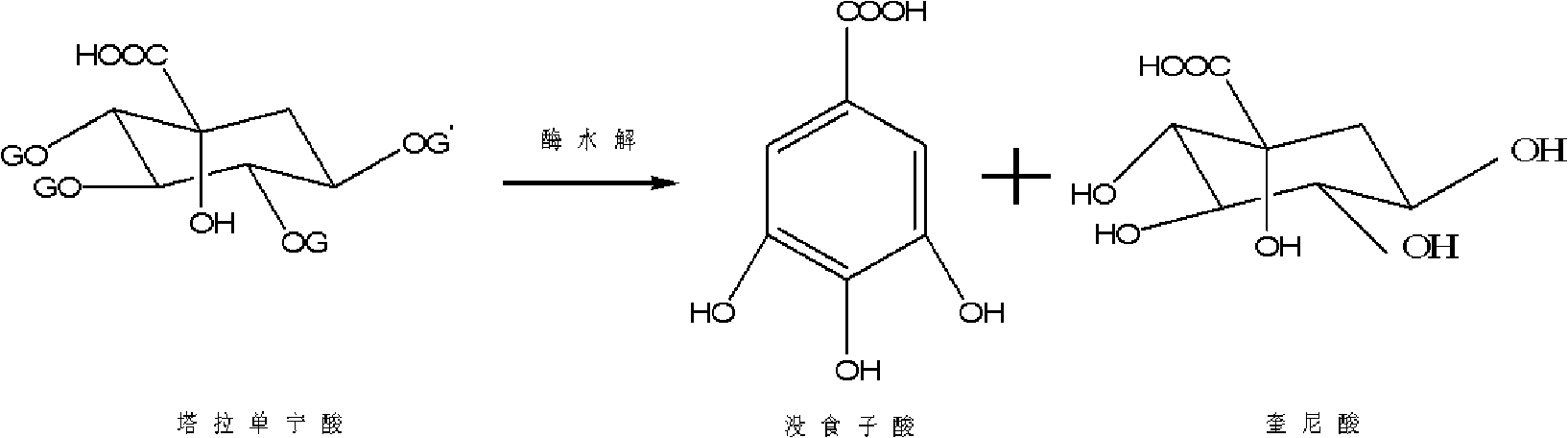
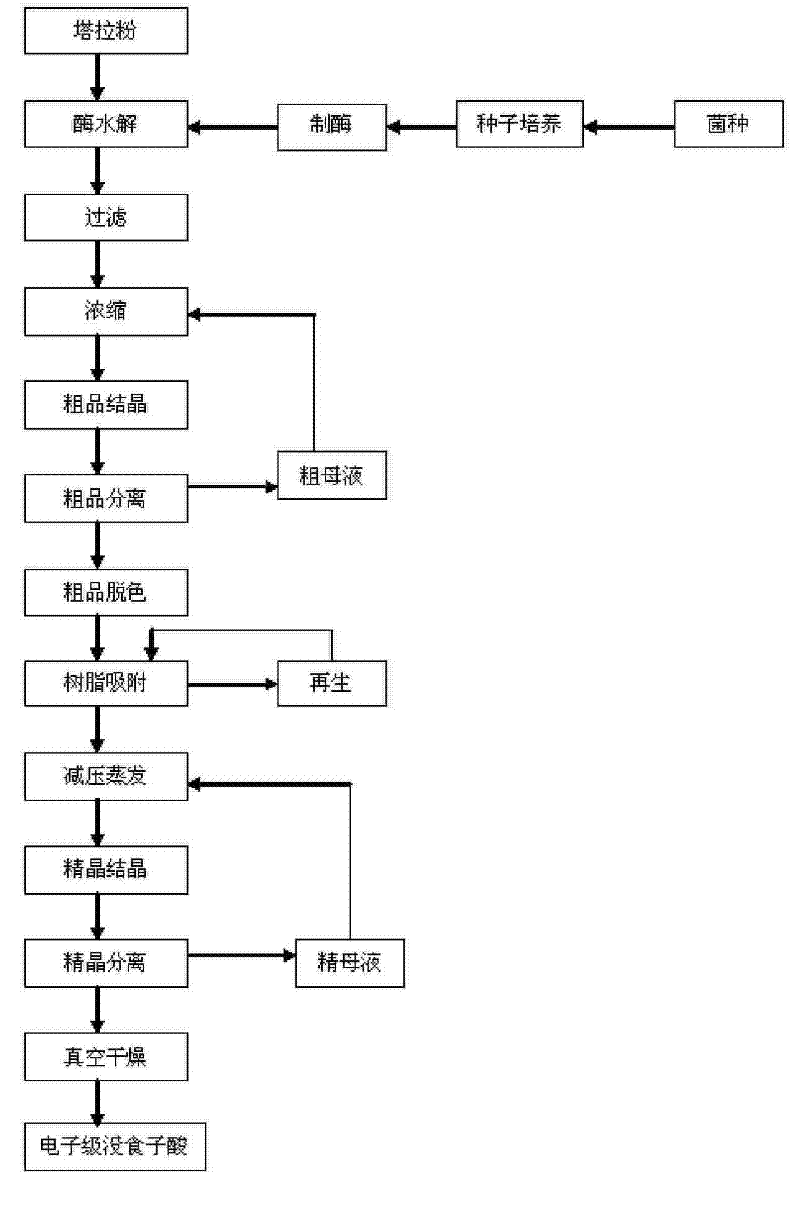
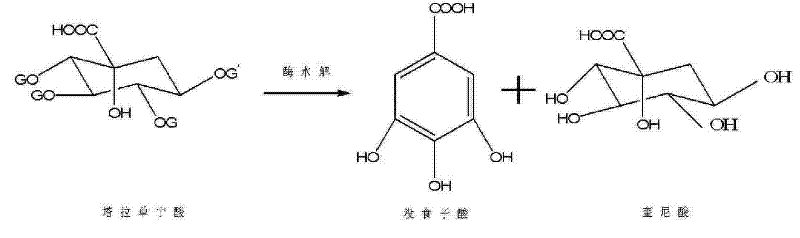
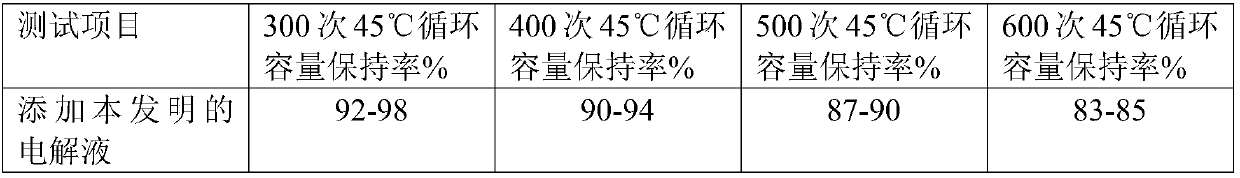
Method for preparing electronic grade gallic acid
ActiveCN101643755AExtended service lifeReduce unit consumptionMicroorganism based processesFermentationMicroorganismGallic acid ester
The invention discloses a method for preparing electronic grade gallic acid by using Tara powder as raw material and combining microorganism fermentation method with resin absorption. The method comprises the following steps: fermenting Tara powder with aspergillus niger to prepare raw gallic acid, decoloring the raw gallic acid in ethanol solution, filtrating to obtain filtrate, sending the filtrate through a anionic-cationic exchange resin column, performing reduced pressure evaporation to recycle ethanol until precipitating crystals, cooling, filtrating to obtain refined gallic acid and performing vacuum drying to obtain the electronic grade gallic acid. The contents of Na, Fe, K, Ca, Mg, Cu, Zn, Al and Mn ions are not more than 50ppb. The method has no pollution, high yield and low cost, the resin can be recycled and the method does not damage resin and is applicable to preparing detergent used to wash integrated circuits in electronic industry.
Owner:先微康新材料科技有限公司
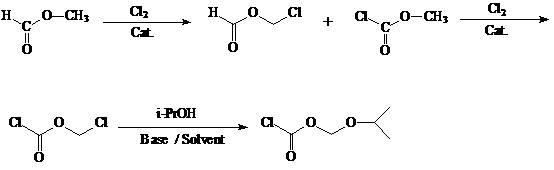
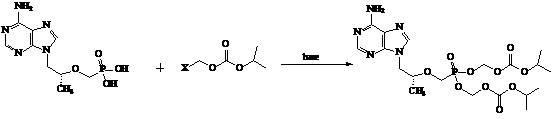
Preparation method of antiviral drug tenofovir disoproxil fumarate intermediate chloromethyl isopropyl carbonate
InactiveCN102399149ARaw materials are safe and easy to obtainHigh yieldOrganic compound preparationCarbonic/haloformic acid esters preparationMethyl formateFormate Esters
The invention discloses a preparation method of antiviral drug tenofovir disoproxil fumarate intermediate chloromethyl isopropyl carbonate, and belongs to the field of antiviral chemical drugs. The preparation method is characterized in that methyl formate as a raw material is synthesized into chloromethyl chloroformate by an one-kettle method and the chloromethyl chloroformate undergoes an esterification reaction to form chloromethyl isopropyl carbonate. The preparation method is easy for operation, has a low cost and a high yield, realizes high purity of products, and is very suitable for industrialized production.
Owner:扬州三友合成化工有限公司
Furfural preparation process
PendingCN111848557ALower reaction yieldEnhanced dehydration selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsProcess engineeringAldehyde
The invention discloses a furfural preparation process. The furfural preparation method adopts a two-step method. The method comprises the following specific steps: firstly, catalyzing hydrolysis of hemicellulose in corncobs by adopting an SO4 < 2-> / Fe2O3-alpha-Al2O3 supported solid acid catalyst; then catalyzing corncob hydrolysis reaction liquid to prepare furfural by using a novel catalyst instead of sulfuric acid, stripping crude aldehyde in the reaction system by using nitrogen instead of water vapor, performing alkali washing and refining on the stripped crude aldehyde by using a continuous centrifugal extraction refining method, introducing the crude aldehyde into a rectifying tower, and rectifying to obtain furfural with the purity of over 99%. According to the method, the furfuralis prepared through the two-step method, lignin and cellulose in the corncob residues can be reserved for reuse, the concentration of the crude aldehyde is increased through nitrogen steam stripping,the furfural production yield is increased, and the wastewater yield is reduced.
Owner:QINGDAO UNIV OF SCI & TECH
Method for synthesizing triallyl phosphite
ActiveCN106967115ALow purityLow yieldGroup 5/15 element organic compoundsFiltrationReaction temperature
The invention discloses a method for synthesizing triallyl phosphite, and belongs to the field of technologies for synthesizing compounds. The method includes adding allyl alcohol and acid binding agents into solvents under the protection of nitrogen, then adding phosphorus trichloride into the solvents and carrying out reaction to obtain reaction liquid; carrying out suction filtration on the reaction liquid and washing, drying, decolorizing and concentrating the reaction liquid to obtain the triallyl phosphite. The allyl alcohol is used as a raw material. The reaction temperatures are lower than -8 DEG C under the control when the phosphorus trichloride is dropwise added into the solvents for the dropwise adding time of 3-4 h, then the temperatures are naturally increased until the temperatures reach 0-30 DEG C, and heat-insulation treatment is carried out for 1-2 h. The method for synthesizing the triallyl phosphite has the advantages that the method is simple and is easy to implement, and the triallyl phosphite prepared by the aid of the method is high in yield and purity.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
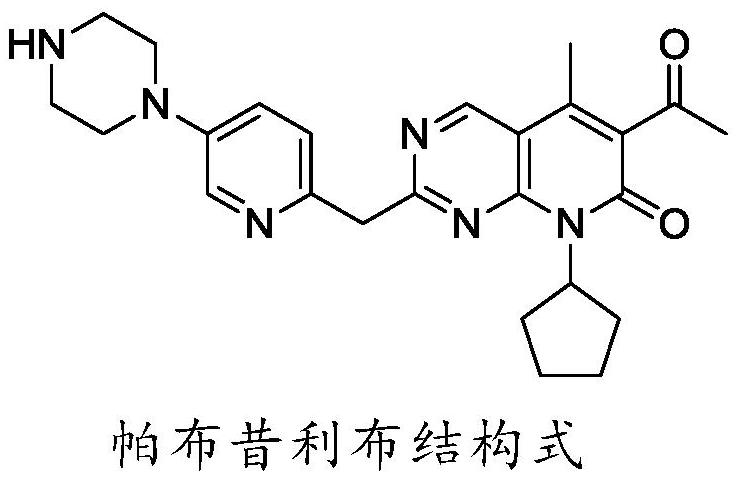
Preparation method of palbociclib parent nucleus structure compound
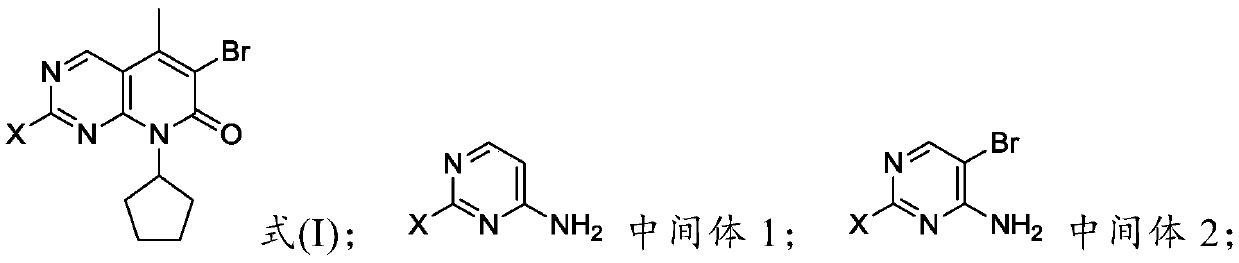
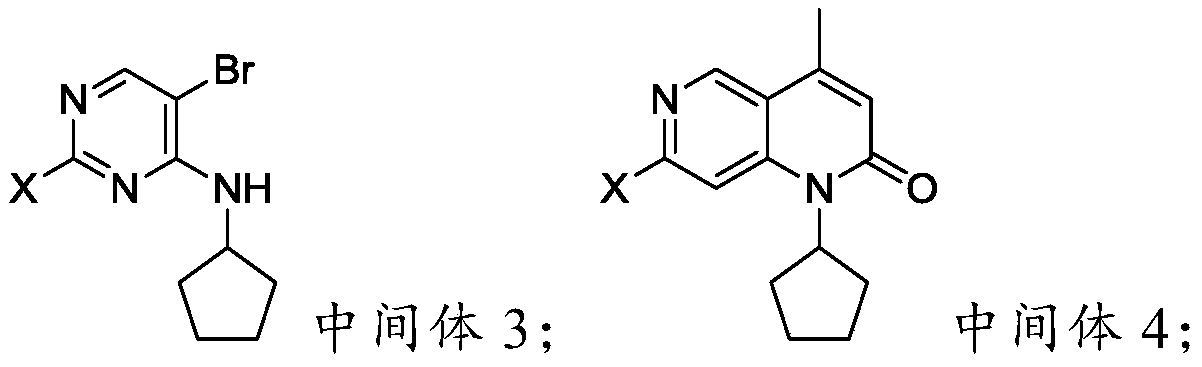
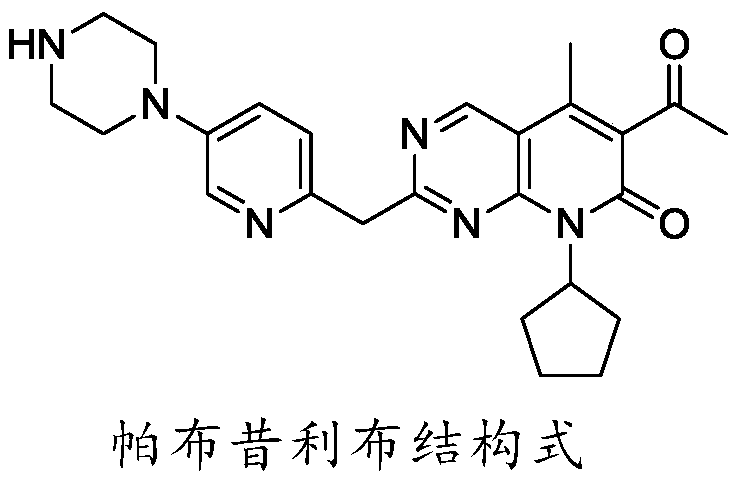
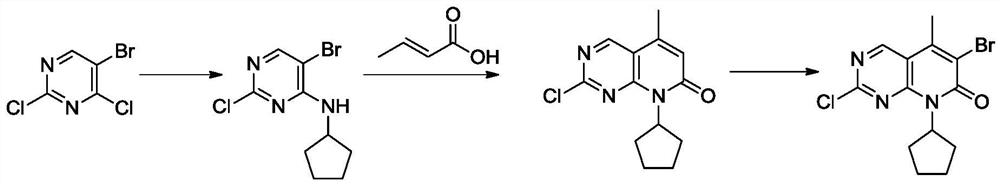
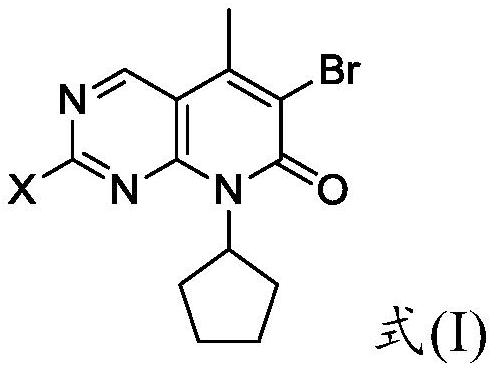
The invention provides a preparation method of a palbociclib mother nucleus structure compound. The preparation method comprises the following step: preparing the palbociclib parent nucleus structurecompound as shown in a formula (I) which is described in the specification by taking cytosine or an intermediate 1 or an intermediate 2 as a starting raw material, wherein the intermediate 1 and the intermediate 2 are as described in the specification, and X is selected from halogen. The method is wide in the source of the starting material, simple in operation process, less in side reaction and high in purity, and accords with the concept of modern green industrial production.
Owner:SHANDONG MAYOHUAWEI TECH CO LTD
N,N-dimethyl tetrahydrofurfuryl amine synthesis method
ActiveCN106349195AMany side effectsSave raw materialsOrganic chemistryChemical recyclingDimethyl formamideFormic acid
The invention relates to organic compound synthesis methods, in particular to an N,N-dimethyl tetrahydrofurfuryl amine synthesis method. The method includes steps: 1) mixing reactants with N,N-dimethyl formamide or N,N-dimethylacetamide and formic acid in a reaction vessel, and performing heating reaction; 1) after heating reaction is finished, performing distillation recovery of DMF (dimethyl formamide) or DMAC (dimethylacetamide) and the formic acid, and subjecting residues to vacuum distillation to obtain N,N-dimethyl furfuryl amine which is an intermediate product; 3) subjecting the intermediate product N,N-dimethyl furfuryl amine to catalytic hydrogenation, and distilling to obtain a product of N,N-dimethyl tetrahydrofurfuryl amine. The N,N-dimethyl tetrahydrofurfuryl amine synthesis method has advantages that raw materials are cheap and extensive in source; problems of low furfural yield and difficulty in separation due to instability of furfural acids are avoided, and acidity and reducibility of the formic acid are fully used; high hydrogenation selectivity and catalyst recyclability are realized; mild conditions, easiness in implementation and high raw material utilization rate are realized, and excessive formic acid and N,N-dimethyl formamide or N,N-dimethylacetamide are easy to recover.
Owner:XIAMEN UNIV
Novel photochromic azobenzene compound and synthesis method thereof
InactiveCN104926684ASimple post-processingImprove conversion rateOrganic chemistryEnvironmental resistanceSynthesis methods
The invention provides a novel photochromic azobenzene compound and a synthesis method thereof and relates to a novel compound N-[4-[2-(4-methoxyphenyl) diazenyl] phenyl]-2-nitroaniline with a photochromic property and a synthesis method of the novel compound. The synthesis method comprises diazonium coupling reaction and coupling reaction between aromatic amine and aromatic halides. According to the invention, coupling reaction conditions of aromatic amine and aromatic halides are very simple, to be specific, only alkali metal fluorides with equal molar mass are added into reaction liquid, and reflux is carried out at 130 DEG for 3 h; a solvent is not required to be subjected to anhydrous anaerobic treatment, that reaction is carried out under nitrogen or argon atmosphere is not required, no side reaction is generated, aftertreatment of the product is simple, the conversion rate is higher, the yield of the target product is 48-74%, in addition, equipment adopted by the invention is relatively simple, the cost is low, and obvious economic benefits and environment-protection benefits are realized. The defects, in the prior art, that coupling reaction between aromatic amine and aromatic halides can be carried out at high temperature, with existence of inorganic base and under anhydrous anaerobic inert atmosphere usually, the reaction time is long, side reaction is multiple and the yield is low are overcome.
Owner:HUBEI UNIV
A method of producing electronic grade gallic acid
ActiveCN101643755BExtended service lifeReduce unit consumptionMicroorganism based processesFermentationGallic acid esterIon-exchange resin
The invention discloses a method for producing electronic-grade gallic acid by using tara powder as a raw material through microbial fermentation and resin adsorption. Tara powder is used as raw material to produce electronic-grade gallic acid by microbial fermentation combined with resin adsorption, and Tara powder is fermented with Aspergillus niger to produce crude gallic acid. Then, the ethanol was evaporated under reduced pressure to recover the ethanol until the crystals were precipitated, and the refined gallic acid was obtained by cooling and filtration, and the electronic grade gallic acid was obtained after vacuum drying. Na, Fe, K, Ca, Mg, Cu, Zn, Al, Mn ions are all ≤50ppb. The method has the advantages of no pollution, high yield, low cost, recyclable resin and no damage to the resin, and is suitable for washing integrated circuits with detergent in the electronic industry.
Owner:先微康新材料科技有限公司
Synthetic method of triallyl phosphate
ActiveCN106957332BLow purityLow yieldGroup 5/15 element organic compoundsPhosphateReaction temperature
The invention provides a method for synthesizing triallyl phosphate, and belongs to the technical field of compound synthesis. The method comprises the following steps: adding allyl alcohol serving as a raw material and an acid-binding agent into a solvent under protection of nitrogen; then, adding phosphorus oxychloride to react; pumping and filtering the reacting liquid; washing, drying, decoloring and concentrating to obtain triallyl phosphate; controlling the reacting temperature to be less than negative 30 DEG C when the phosphorus oxychloride is dropwise added for 3.4-7.5 hours; and naturally heating to -2-zero DEG C, and stopping reaction. The synthesizing method is simple and easy to operate, and the prepared triallyl phosphate has high yield and high purity.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
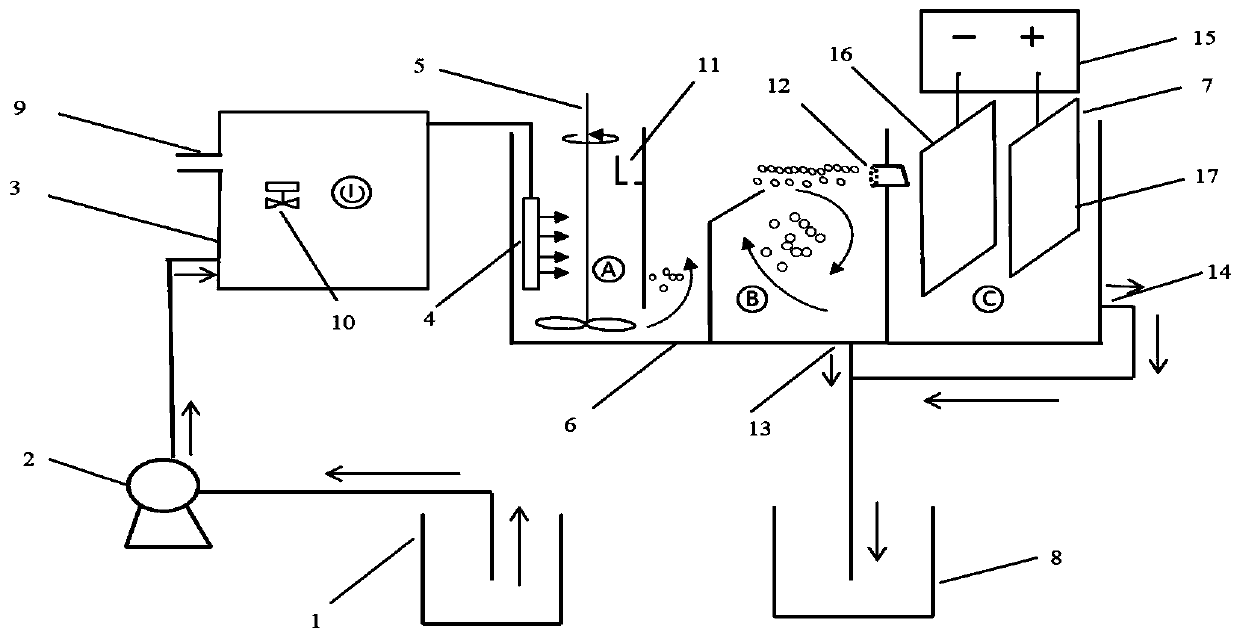
Method for removing perfluorinated compounds (PFCs) in wastewater through micro-nano gas-floating coupled electrooxidation device
InactiveCN110342728AReduce dosageLarge amount of water to be treatedWater contaminantsTreatment with aerobic and anaerobic processesMicro nanoElectrolysis
The invention relates to a method for removing perfluorinated compounds (PFCs) in wastewater through a micro-nano gas-floating coupled electrooxidation device. The method comprises the steps that (S1)a water inlet pump and a micro-nano bubble generation device are started, the gas inlet amount is adjusted to be 0.3-0.7 mL / min, and a milky gas-water mixture reaches a contact chamber of a reactiondevice through a high-speed water outlet diffuser; (S2) a stirrer is started, the pH value of the wastewater is adjusted through acid or base firstly through a chemical adding groove, and then a coagulant is added; (S3) the gas-water mixture reaches a separation chamber subsequently, the PFCs in the wastewater float upwards to the water surface under the effect of coercing and jacking of bubbles and the effect of a small amount of flocculant, and enter an electrooxidation unit from a slag-water mixture outlet, and the wastewater not containing the PFCs is discharged from a water outlet of a separation chamber; (S4) an electrolyte is added into the electrooxidation unit, after a collected PFCs water-slag mixture reaches a certain volume, the current density is adjusted for electrolysis, andpurified water is discharged from the water outlet; and (S5) the wastewater discharged from the water outlet finally enters a water outlet groove and is discharged after reaching the standard.
Owner:江苏智诚达环保科技有限公司
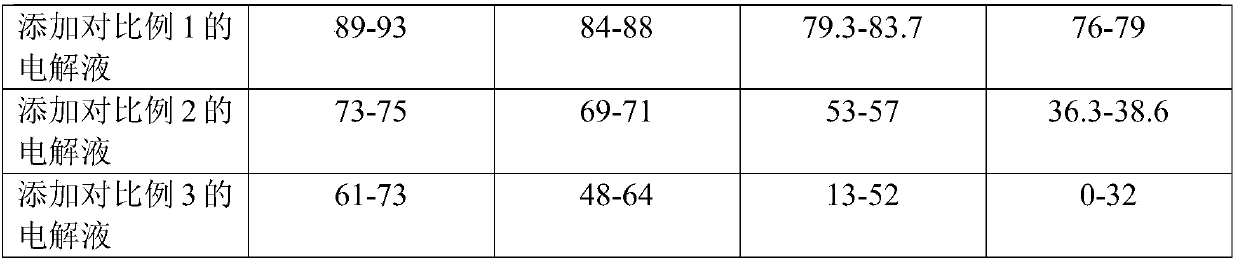
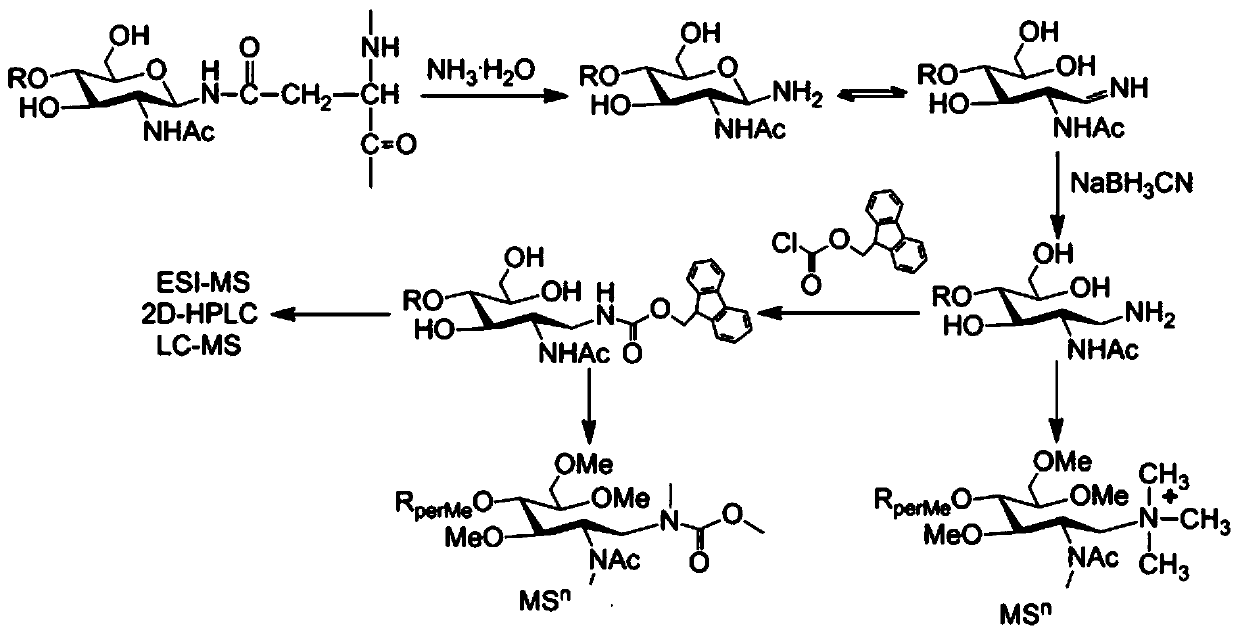
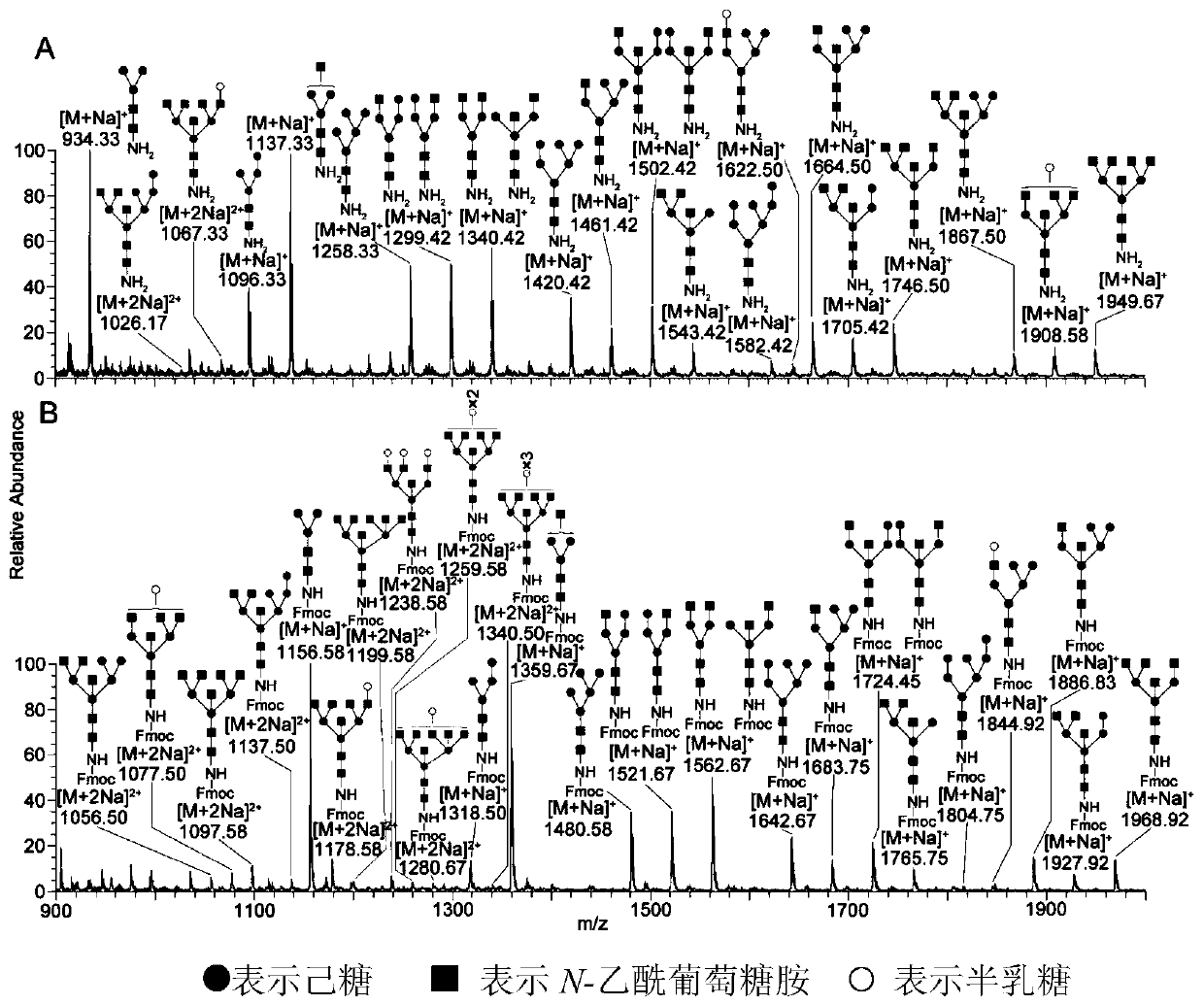
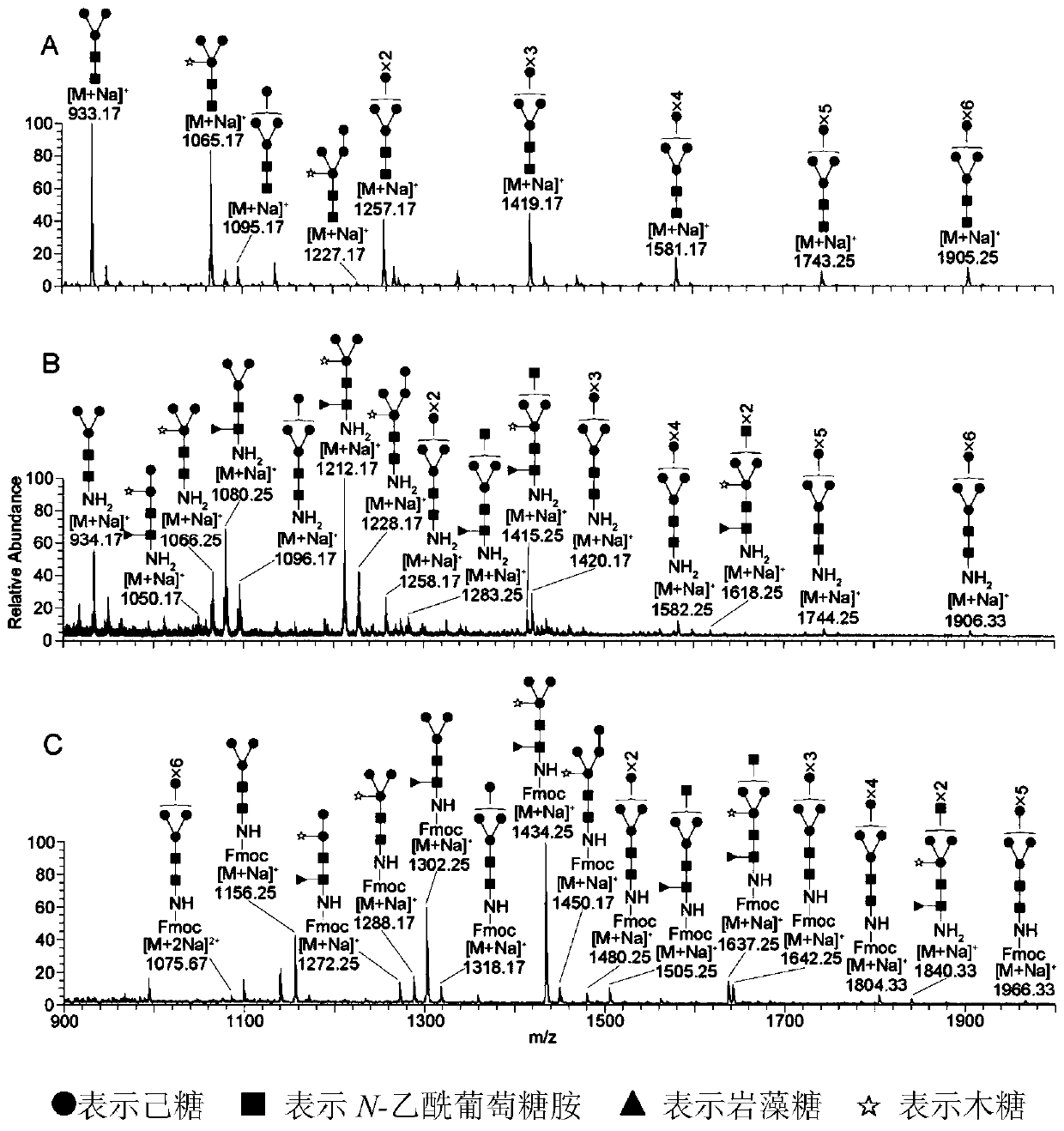
Method for separation, analysis and identification of reductively released glycoprotein n-glycan and its derivatives
ActiveCN107389805BImprove general performanceAvoid peeling degradationComponent separationMaterial analysis by electric/magnetic meansFucosylationSugar amine
The invention belongs to the field of glycobiology technology, and specifically relates to a method for the preparation, separation, analysis and identification of N-sugar chains of reductively released glycoproteins and their derivatives. The method for reductively releasing glycoprotein N-sugar chains of the present invention is to first dissolve the glycoprotein in concentrated ammonia water to hydrolyze the sugar chains, and then pass it through NaBH 3 The reduction of CN yields an active amino group (NH 2 ) of sugar amines, this solution is highly versatile and is suitable for neutral N-glycans, acidic N-glycans and neutral N-glycans containing core α-1,3-fucosylation, releasing N-sugar chains are highly stable and will not degrade, allowing direct preliminary analysis. In addition, the present invention provides a method for labeling the obtained N-sugar chain with Fmoc, and also provides a method for separating, analyzing and identifying the N-sugar chain and the Fmoc-labeled N-sugar chain.
Owner:NORTHWEST UNIV
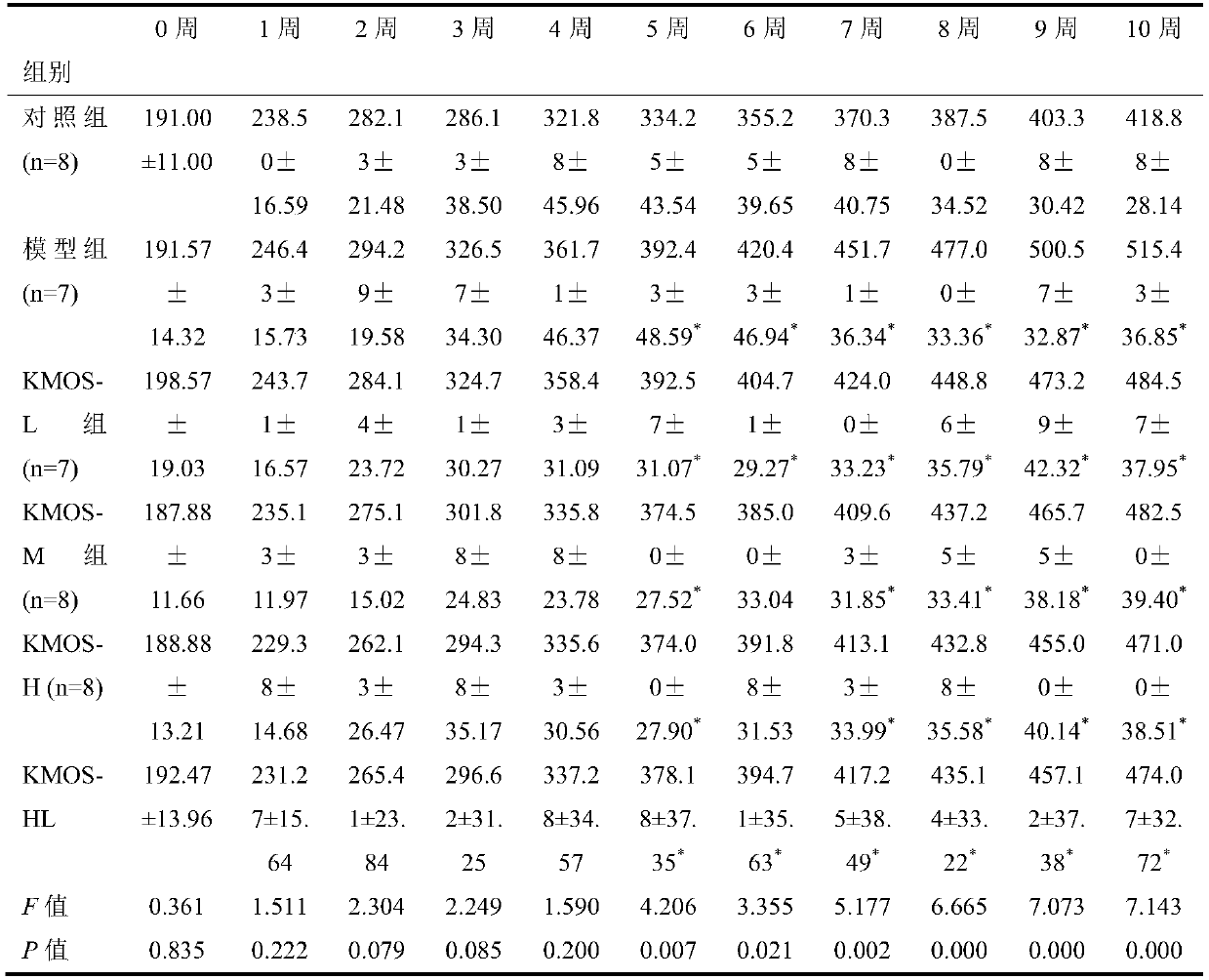
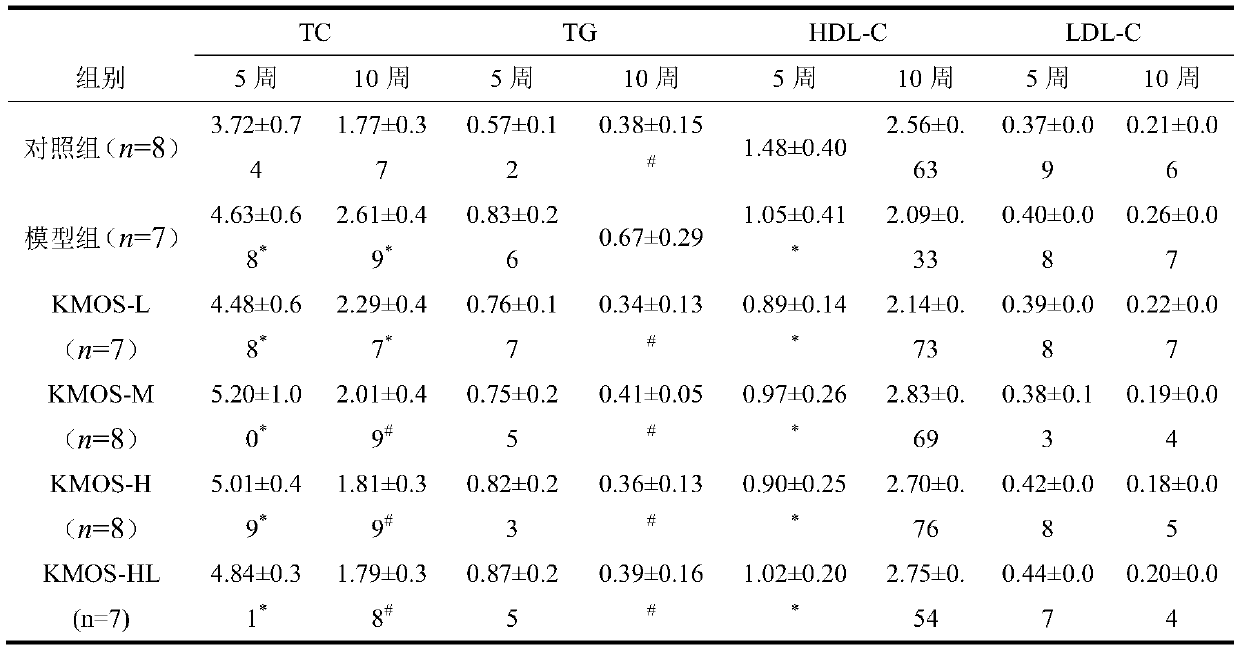
A kind of enteral nutrition preparation combining konjac manna oligosaccharide and L-glutamine and its application
ActiveCN107929292BMany side effectsRegulate blood sugarOrganic active ingredientsMetabolism disorderEnteral Nutrition PreparationNutrition
The invention belongs to the technical field of medicines, and particularly discloses an enteral nutrition preparation containing konjac mannan oligosaccharide and L-glutamine and application thereofin hyperlipoidemia nutritional therapy. When the method is adopted in the nutritional therapy of a hyperlipoidemia rat model, a certain curative effect is realized, no toxic and side effects exist, the preparation method is simple, the preparation raw materials are easy to obtain, and the application prospect is wide.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
Method for synthesis of long-chain fatty acid ester derivative
ActiveCN110627642AMany side effectsSimple post-processingOrganic compound preparationCarboxylic acid esters preparationPalmitatesEthyl acetate
The invention relates to a method for synthesis of a long-chain fatty acid ester derivative. Specifically, a hydrochloride of glycine methyl ester or glycine ethyl ester is used as a catalyst to catalyze the esterification reaction of long-chain fatty acid. The method includes: subjecting alcohol and long-chain fatty acid to esterification reaction under the action of the catalyst at certain temperature condition, then conducting extraction and precipitation with ethyl acetate, performing flushing with a sodium chloride aqueous solution for purification. A hydrochloride of glycine methyl esteror glycine ethyl ester is adopted as the catalyst, which belongs to a green catalyst, is the development trend of modern chemistry, has the characteristics of no corrosion to the reaction kettle, lowprice, no toxicity and the like, and is suitable for use as a catalyst to produce palmitate and laurate perfume raw materials.
Owner:PANASIA OLAUGHLIN BIO TECH WUHAN CO LTD
A kind of preparation method of palbociclib mother core structure compound
ActiveCN111362939BWide variety of sourcesLow priceOrganic chemistryBiochemical engineeringCombinatorial chemistry
The present invention provides a preparation method of palbociclib parent core structure compound, which comprises preparing palbociclib mother represented by formula (I) using cytosine or intermediate 1 or intermediate 2 as starting material Core structure compound; Intermediate 1; Intermediate 2; wherein X is selected from halogen. The method of the invention has wide source of starting materials, simple operation process, few side reactions and high purity, and conforms to modern green industrial production.
Owner:SHANDONG MAYOHUAWEI TECH CO LTD
Enteral nutrition preparation containing konjac mannan oligosaccharide and L-glutamine and application
ActiveCN107929292AMany side effectsRegulate blood sugarOrganic active ingredientsMetabolism disorderEnteral Nutrition PreparationMedicine
The invention belongs to the technical field of medicines, and particularly discloses an enteral nutrition preparation containing konjac mannan oligosaccharide and L-glutamine and application thereofin hyperlipoidemia nutritional therapy. When the method is adopted in the nutritional therapy of a hyperlipoidemia rat model, a certain curative effect is realized, no toxic and side effects exist, the preparation method is simple, the preparation raw materials are easy to obtain, and the application prospect is wide.
Owner:WUHAN GENERAL HOSPITAL OF GUANGZHOU MILITARY
The synthetic method of triallyl phosphite
ActiveCN106967115BLow purityLow yieldGroup 5/15 element organic compoundsFiltrationReaction temperature
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
Preparation method of N-formyl cefotaxime
The invention discloses a preparation method of N-formyl cefotaxime, which is characterized by comprising the following steps: adding anhydrous formic acid into acetic anhydride, controlling the temperature at 25-35 DEG C, reacting for 30 minutes, cooling to 15 DEG C, adding cefotaxime acid in batches, heating to 35-40 DEG C, adding a metal catalyst to continue reacting when reactants start to agglomerate, reacting for 30 minutes, adding purified water, and reacting for 30 minutes to obtain N-formyl cefotaxime. And adjusting the pH value when white crystals are separated out, filtering and washing when the temperature is reduced to 10-15 DEG C, and drying at the temperature of 50-60 DEG C and the pressure of-0.085--0.095 Mpa until the water content is less than 2.5% to obtain a target product. According to the method, multi-step synthesis by taking 7-ACA as a raw material is not needed, the production cost is effectively reduced, the reaction steps are simple, the steps of phase inversion purification, carbon decoloration and the like are not needed, the operation is simple and easy to control, the weight yield can reach 80%, and the highest product purity can reach 98.6%. The method fills the blank of N-formyl cefotaxime impurities prepared by chemical synthesis, and has important theoretical significance and practical application value for improving the quality of cefotaxime sodium and reducing the clinical medication risk.
Owner:河北合佳创新医药科技有限公司
Synthesizing method of 2-butyl-1-octanol
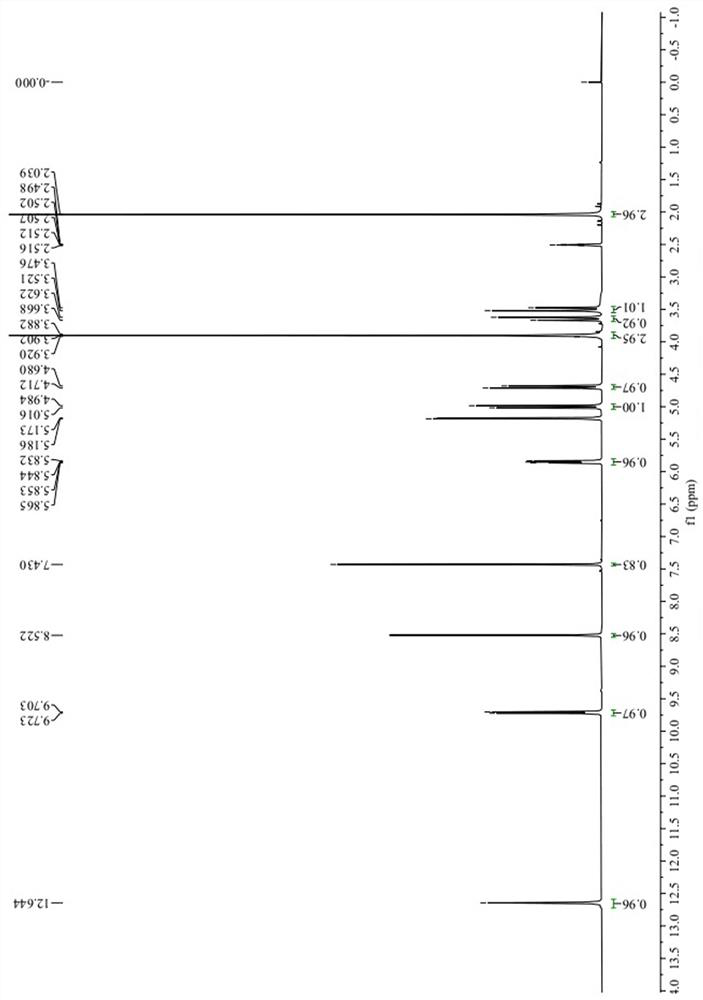
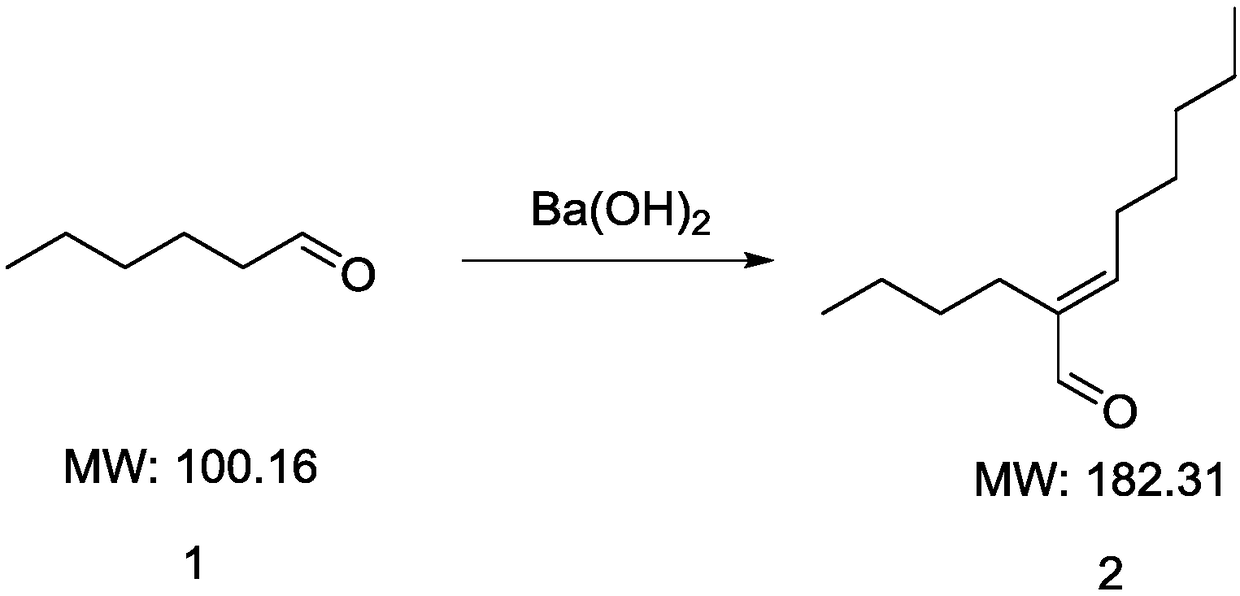
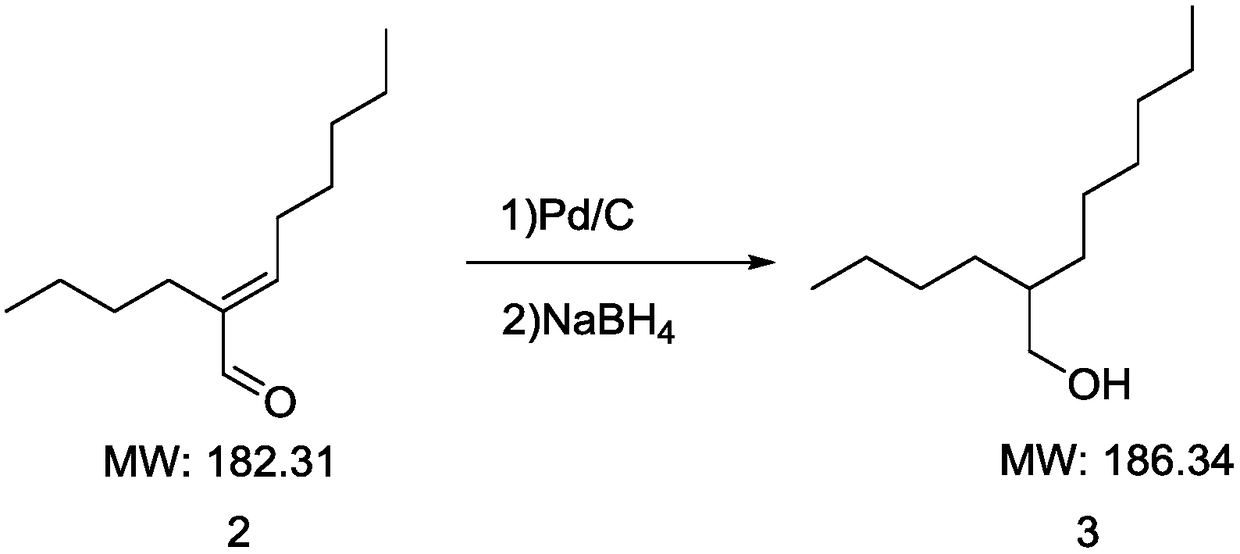
InactiveCN108191607AQuick responseMany side effectsOrganic compound preparationPreparation by hydrogenationPalladium on carbon2-butyl-2-octenal
The invention discloses a synthesizing method of 2-butyl-1-octanol. The method includes the following steps of conducting an aldol condensation reaction with n-caproaldehyde as the initiator to obtain2-butyl-2-octenal, wherein a catalyst in the aldol condensation reaction is barium hydroxide; conducting a primary reduction reaction on 2-butyl-2-octenal to obtain a mixture of aldehyde and alcohol,and conducting a secondary reduction reaction on the mixture of aldehyde and alcohol to obtain 2-butyl-1-octanol, wherein a reducing agent in the primary reduction reaction is palladium on carbon, and a reducing agent in the secondary reduction reaction is sodium borohydride. The method is easy to operate, few byproducts are produced, yield is high, the requirements for devices are not high, andindustrial production is easy.
Owner:福建未来药业有限公司
A method for synthesizing long-chain fatty acid lipid derivatives
ActiveCN110627642BMany side effectsSimple post-processingOrganic compound preparationCarboxylic acid esters preparationPtru catalystEthyl acetate
The invention relates to a method for synthesizing long-chain fatty acid lipid derivatives. Specifically, the hydrochloride of glycine methyl ester and glycine ethyl ester is used as a catalyst to catalyze the esterification reaction of long-chain fatty acids, including alcohol, long-chain The fatty acid is esterified under the action of a catalyst at a certain temperature, and then extracted with ethyl acetate, washed and purified with an aqueous solution of sodium chloride, and the hydrochloride of glycine methyl and ethyl esters is used as a catalyst. It is a green catalyst and is The development trend of modern chemistry has the characteristics of non-corrosive reaction kettle, low price, non-toxic, etc. It is suitable as a catalyst to produce palmitate and laurate fragrance raw materials.
Owner:PANASIA OLAUGHLIN BIO TECH WUHAN CO LTD
Specific indole compound and its preparation and use in treating and preventing cancers
InactiveCN1199946CPrevent proliferationInduce apoptosisOrganic active ingredientsNervous disorderCancer preventionHuman tumor
The invention relates to a series of organic heterocyclic compounds, JN-2518 series compounds and their preparation methods and their application in treating and preventing cancer and other diseases. The series of compounds are prepared through organic synthesis reactions and are used in the field of medicine. This series of compounds can specifically inhibit the activity of cell cycle kinases, block cell proliferation, and induce cell apoptosis, thereby effectively inhibiting the growth of various human tumor cells including colon cancer, prostate cancer, breast cancer, and neuroblastoma. The series of compounds have low toxicity and side effects, and can be used to treat various diseases of humans and animals, including the treatment and prevention of malignant tumors, senile dementia, psoriasis, cardiovascular diseases, glomerulonephritis and AIDS.
Owner:JC (WUXI) CO INC
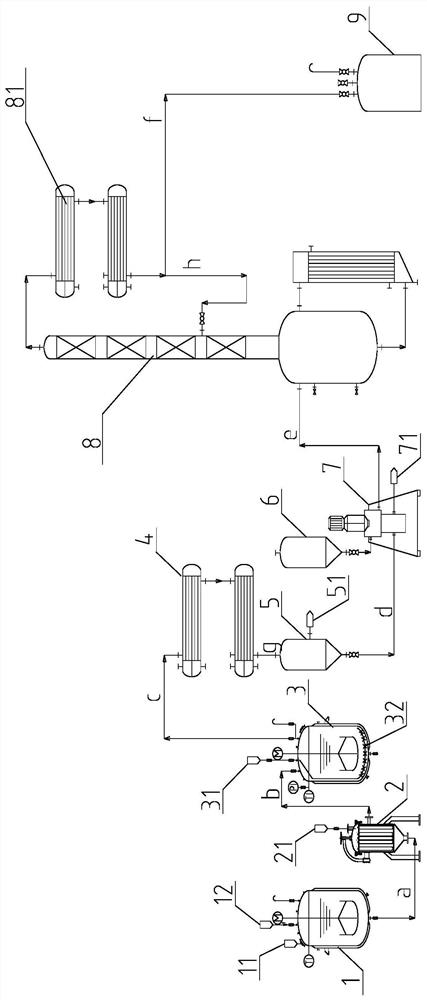
Process for producing 7-amino-3-[(1-methyl pyrrolidine) methyl]-3- cephalosporin-4-carboxylic dihydrochloride
InactiveCN101429207BReduce decompositionIncreased weight yieldOrganic chemistryHydrolysateCarboxylic acid
The invention discloses a method for preparing 7-amino-3-[(1-methylpyrro-lidinio)methyl]-3-cephem-4-carboxylic acid dihydrochloride, which comprises the following steps: imidazole, trimethylchlorosilane and 7-ACA react in a methylene dichloride solvent at a temperature of between 10 and 30 DEG C, and then are filtered; the temperature is reduced, and TMSI is dripped to react; THF is added into the mixture after methylene dichloride is distilled from a reaction feed liquid, and a N-methylpyrrolidine solution is dripped and stirred at a temperature of between 30 DEG C below zero and 25 DEG C below zero to react; methanol is dripped after the reaction, and then concentrated hydrochloric acid or hydriodic acid is dripped to hydrolyze; a hydrolysate is added with the methylene dichloride, and an aqueous phase is obtained through the separation; a yellow aqueous solution is obtained after the aqueous phase is decolored; and then acetone is added into the mixture to be filtered, washed and dried to obtain a 7-MPCA product. The methylene dichloride is distilled, so as to effectively improve the utilization rate of equipment, and the method can improve 30 percent of the utilization rate ofthe equipment compared with a process that the methylene dichloride is not distilled; and imidazole hydrochloride generated by the reaction which affects the subsequent layering and crystallization is filtered, which is favorable for the reaction, and ensures that the yield can reach 100 percent and the purity of a liquid phase is more than 99 percent.
Owner:河北九派制药股份有限公司
A kind of preparation method of sucrose-6-acetate
ActiveCN111205340BLow yieldMany side effectsEsterified saccharide compoundsSugar derivativesPtru catalystSucrose
The invention discloses a preparation method of sucrose-6-acetate. Under the action of a supported platinum catalyst, sucrose and 1-acetyl-1H-1,2,3-triazol[4,5-B]pyridine Esterification occurs with high selectivity to generate sucrose-6-acetate, and the product is separated by crystallization in solution; the supported platinum catalyst is expressed as Pt / X / Z-Y, wherein X is selected from N-hydroxyl-ortho Phthalimide, 1-Hydroxybenzotriazole, N-Hydroxymaleimide, N-Hydroxysuccinimide, 2,2,6,6-Tetramethylpiperidine Oxide, Y It is selected from neutral alumina, silica, molecular sieve, kaolin, and Z is selected from pinacol diborate, biscatechol borate, diphenyltetramethylsilane, and hexamethylsilane. The process of the invention avoids the problems of the use of highly toxic reagents and difficulty in solvent recovery in the prior art, and the conversion rate of raw materials and product selectivity can reach more than 90%.
Owner:WANHUA CHEM GRP CO LTD
A kind of method of producing 4-propyl vinyl sulfate
A method for producing 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide comprises an addition reaction, an oxidation reaction and a purifying process. The method concretely comprises the following steps: 1, carrying out the addition reaction: carrying out addition reaction on thionyl chloride and 1,2-pentanediol as reaction raw materials; 2, carrying out the oxidation reaction: adding a sodium hypochlorite and catalyst mixed solution, carrying out the oxidation reaction to obtain a water phase and organic phase co-existence reaction solution, standing the water phase and organic phase co-existence reaction solution for layering, and separating the obtained water phase to obtain the organic phase which is crude 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide; and 3, purifying: carrying out molecular distillation to obtain 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide. The purity of the prepared 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide can be greater than 99.5%, the water content is not greater than 100PPM, the acid value is not greater than 100PPM, and 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide can be added to a battery in order to improve the performances of the battery and prolong the life of the battery.
Owner:SHIJIAZHUANG SAN TAI CHEM CO LTD
A kind of powdery pd/sio2 catalyst and its preparation method and application
ActiveCN103566932BReduce adsorptionMany side effectsPreparation by hydrogenationMetal/metal-oxides/metal-hydroxide catalystsPotassiumManganese
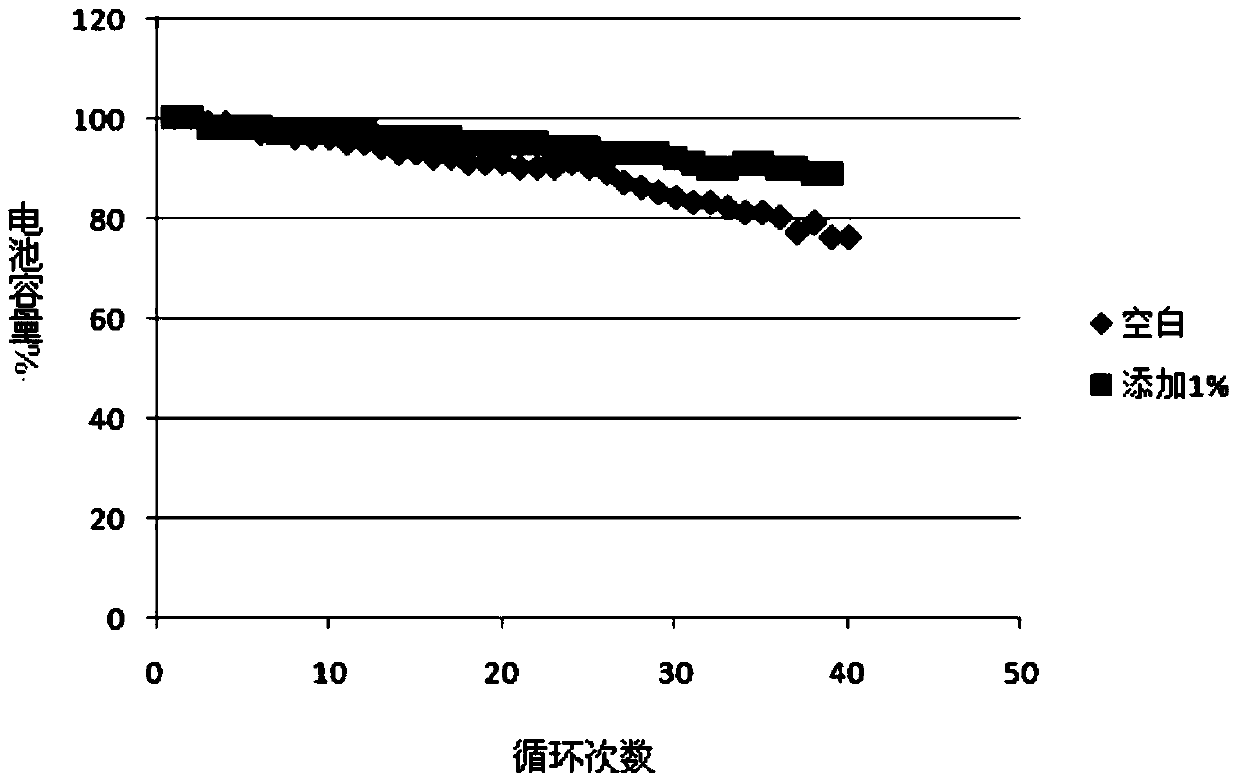
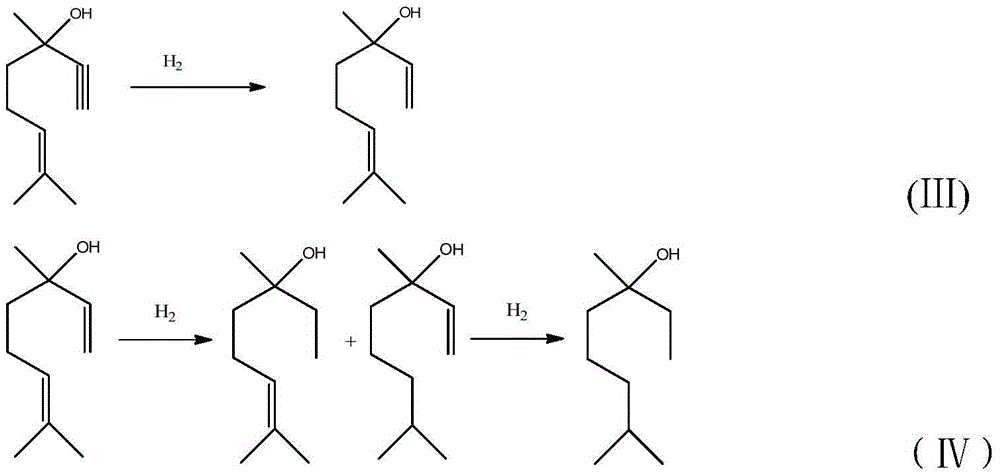
The invention discloses a powdered Pd / SiO2 catalyst, which is composed of the following components in percentage by weight: 90-99.98% of silica gel powder, 0.01-5% of a palladium metal component and 0.01-5% of a cocatalyst, wherein the cocatalyst is one or more of lithium, sodium, potassium, chromium, manganese, zinc, silver, titanium, lead and bismuth, and the granularity of the silica gel powder is 150-500 meshes. The catalyst is applied to the hydrogenation reaction of alkynol compounds with a large molecular weight in a reaction kettle, and can eliminate the defects that a currently used lindlar catalyst is strong in alkalinity, more in side effects such as isomerism and polymerization and the like, high in leftovers rate, small in specific surface, low in adsorptivity, poor in selectivity, and the like. The invention also discloses a preparation method of the powdered Pd / SiO2 catalyst and the application of the powdered Pd / SiO2 catalyst in the hydrogenation reaction of alkynol compounds. While the powdered Pd / SiO2 catalyst is applied to the hydrogenation reaction of alkynol compounds so as to produce enol compounds, the powdered Pd / SiO2 catalyst is high in conversion rate and selectivity.
Owner:SHANDONG NHU PHARMA +1
A kind of n, n-dimethyltetrahydrofurfurylamine synthetic method
ActiveCN106349195BWide variety of sourcesIncrease profitOrganic chemistryChemical recyclingSimple Organic CompoundsSynthesis methods
Owner:XIAMEN UNIV
Traditional Chinese medicine composition for treating dysentery and preparing method thereof
InactiveCN106176917AEasy to prepareImprove extraction efficiencyDigestive systemAgainst vector-borne diseasesAlcohol contentMixed materials
The invention discloses a traditional Chinese medicine composition for treating dysentery. The traditional Chinese medicine composition is prepared from, by weight, 300-410 parts of ludwigia prostrate and 185-215 parts of radix sophorae flavescentis. The preparing method of the traditional Chinese medicine composition includes the following steps that 1, the raw materials are broken and mixed; 2, the mixed materials are decocted three times, the mixed materials are decocted for 2 h for the first time, for 1.5 h for the second time and for 1 h for the third time, and decoction is converged; 3, the decoction is filtered and concentrated to clear paste with the relative density of 1.08 at the temperature of 80 DEG C; 4, the clear paste is cooled, ethyl alcohol is added so that the alcohol content of the clear paste reaches 50-55%, the mixture is stirred uniformly and stands for 48 h, then supernate is taken and filtered, ethyl alcohol is recovered from filter liquor until alcohol smell is eliminated, and the filter liquor continues to be concentrated to clear paste with the relative density of 1.08 at the temperature of 80 DEG C; 5, the clear paste is dried in a belt type vacuum mode, smashed and granulated.
Owner:华润三九(郴州)制药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Method for producing 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide Method for producing 4-propyl-[1,3,2]dioxathiolane-2,2-dioxide](https://images-eureka.patsnap.com/patent_img/93bc29aa-fe83-41af-a96c-e8d9f68ba748/HDA0000728080960000011.PNG)





![Process for producing 7-amino-3-[(1-methyl pyrrolidine) methyl]-3- cephalosporin-4-carboxylic dihydrochloride Process for producing 7-amino-3-[(1-methyl pyrrolidine) methyl]-3- cephalosporin-4-carboxylic dihydrochloride](https://images-eureka.patsnap.com/patent_img/e91e34f4-6619-4094-95c3-8ff6e4d0413a/GSB00000342147200011.PNG)
![Process for producing 7-amino-3-[(1-methyl pyrrolidine) methyl]-3- cephalosporin-4-carboxylic dihydrochloride Process for producing 7-amino-3-[(1-methyl pyrrolidine) methyl]-3- cephalosporin-4-carboxylic dihydrochloride](https://images-eureka.patsnap.com/patent_img/e91e34f4-6619-4094-95c3-8ff6e4d0413a/GSB00000342147200031.PNG)