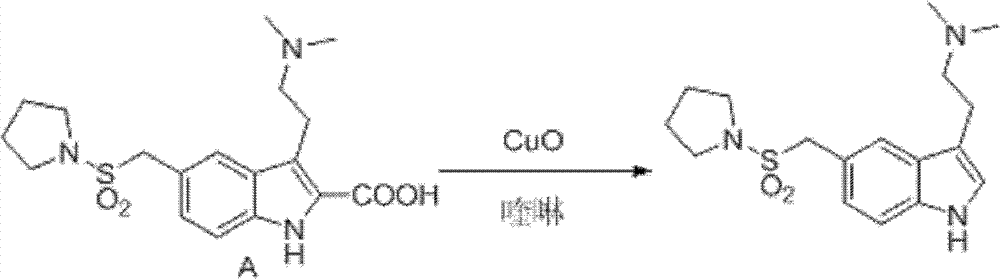
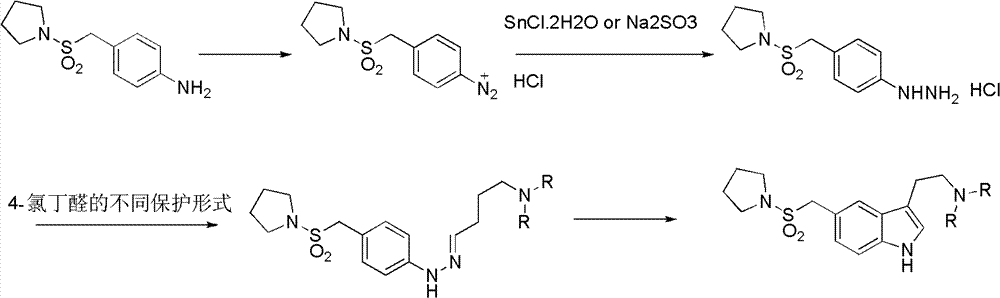
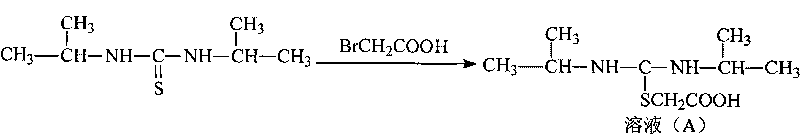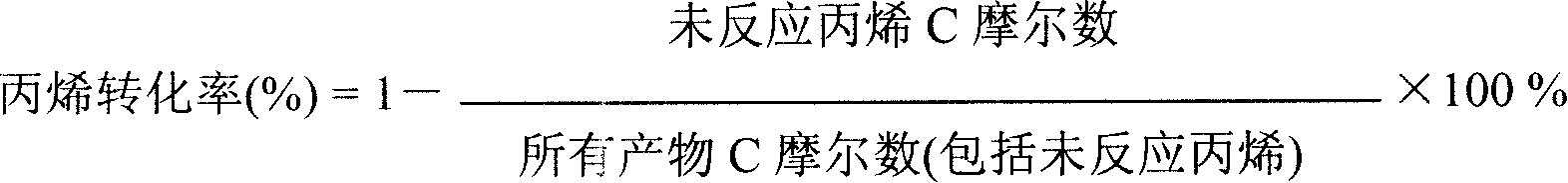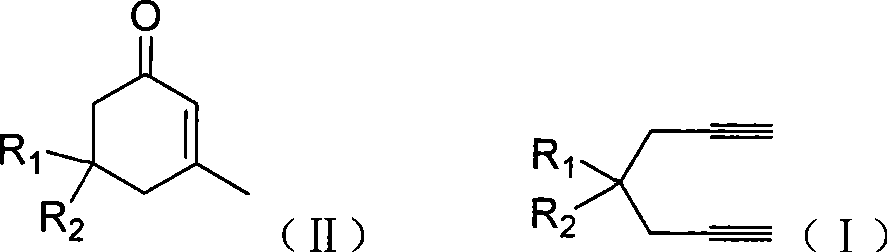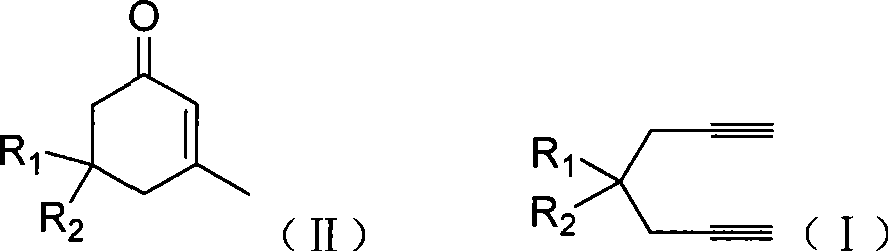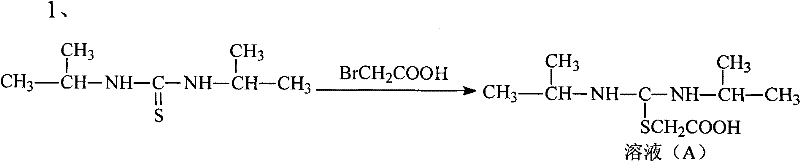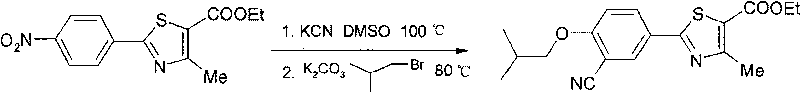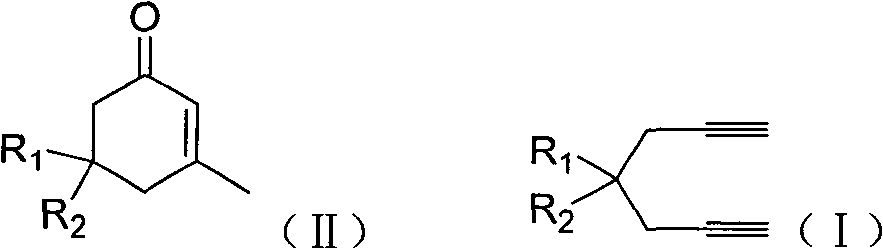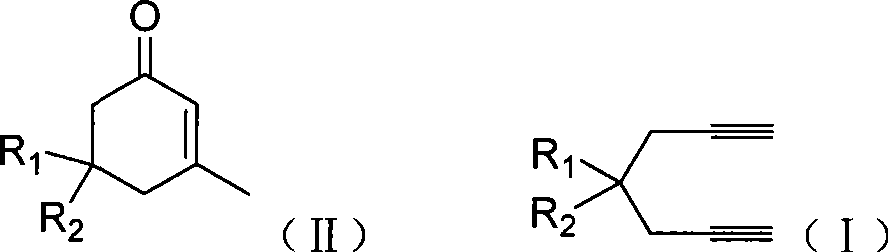Patents
Literature
82results about How to "Lower reaction yield" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
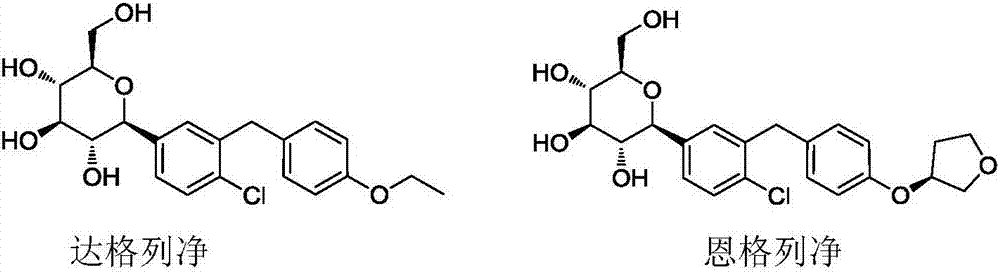
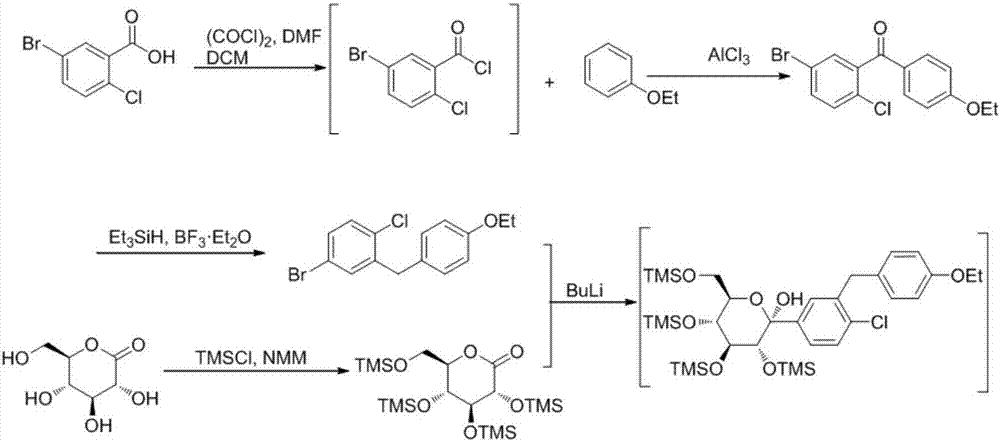
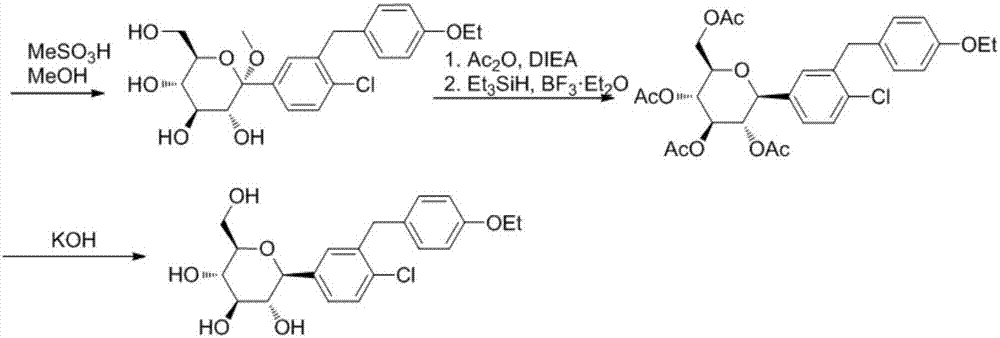
Preparation methods of SGLT-2 diabetes inhibitors and intermediates thereof
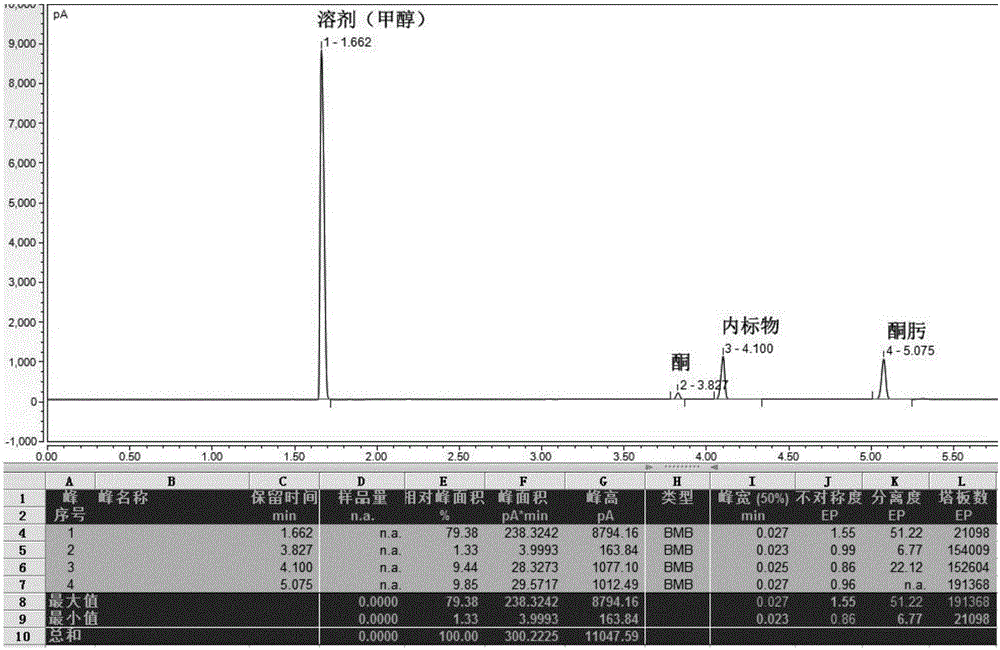
ActiveCN107163092AAvoid it happening againLower reaction yieldSugar derivativesSugar derivatives preparationSynthesis methodsCombinatorial chemistry
The invention provides a preparation method of an intermediate compound 7 of an SGLT-2 diabetes inhibitor dapagliflozin and an intermediate compound 8 of a SGLT-2 diabetes inhibitor empagliflozin, and new synthesis method of two final products. The preparation method comprises the following steps: carrying out carbonyl group reduction and hydroxyl group protection on a (5-halo-2-chlorophenyl)(4-ethoxyphenyl)ketone compound 1 used as an initial raw material to obtain a Grignard addition reaction key compound 4, and carrying out Grignard addition and acetylation to obtain the compound 7 and the compound 8. The dapagliflozin and the empagliflozin are respectively prepared from the compound 8. The methods have the advantages of simplicity in operation, high yield, high purity of the obtained products, and suitableness for amplified production.
Owner:山东科巢生物制药有限公司
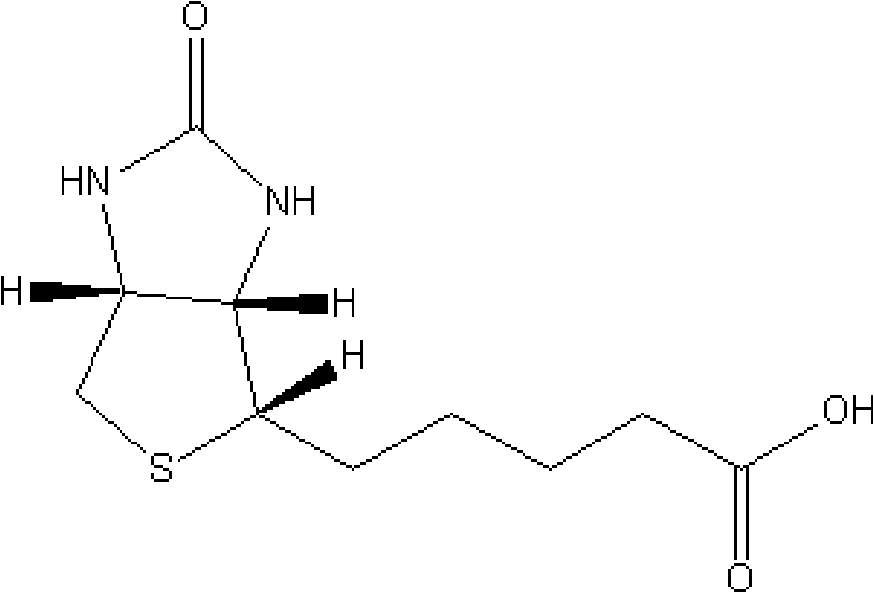
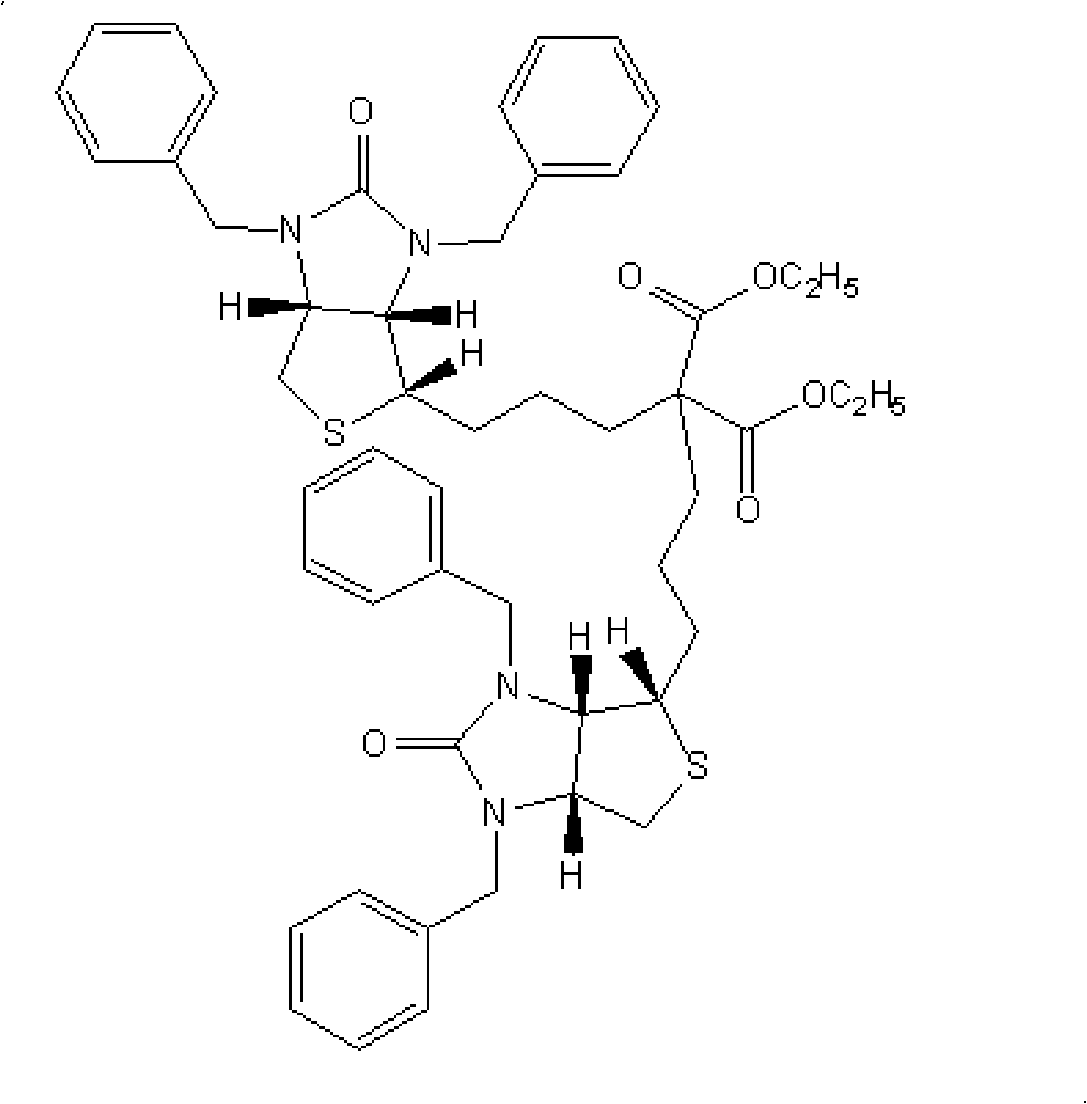
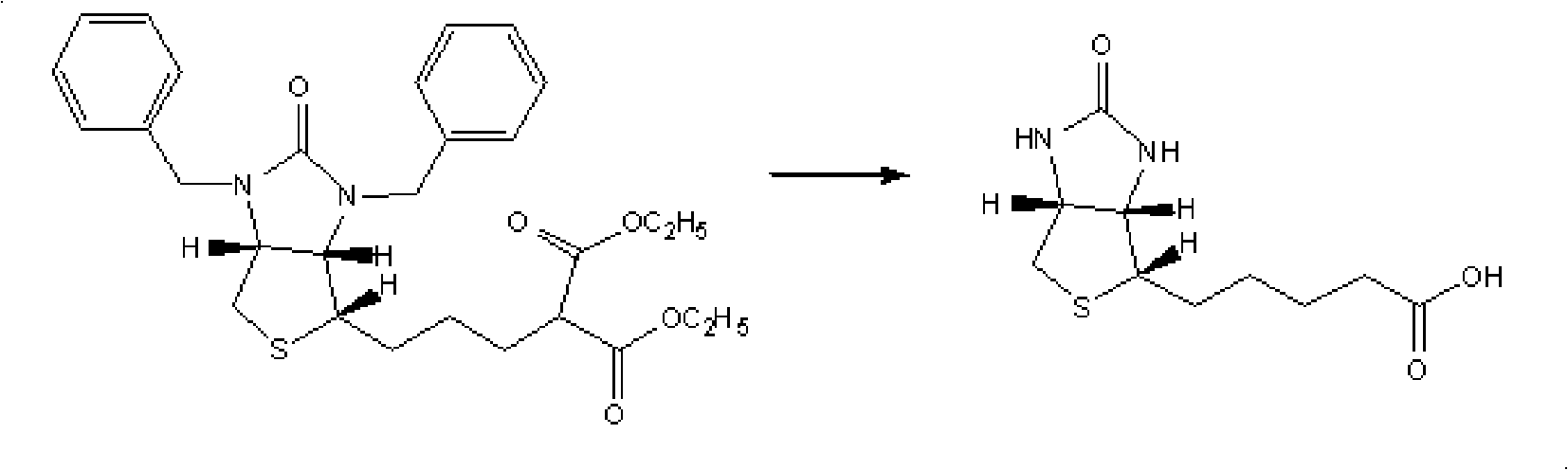
Preparation method of d-biotin
The invention discloses a preparation method of d-biotin. Existing d-biotins can be obtained from debenzylation reactions of bisbenzylbiotin, and can be obtained from debenzylation and decarboxylation reactions of bisbenzylbiotin diester. However, d-biotins obtained from the two methods are not ideal. The preparation method of d-biotin provided by the invention comprises the following steps that: (1) sulfonium halide is adopted as a raw material, and is condensed with 2,2-diethyl acetoacetate, such that bisbenzylbiotin diethyl ester is obtained; the bisbenzylbiotin diethyl ester is processed through debenzylation, hydrolysis, decarboxylation and loop opening reactions; (2) d-biotin which is not processed form the loop opening reaction is separated from the product obtained from the previous step; d-biotin-separated ring-opened object is cyclized with triphosgene, such that d-biotin is obtained. According to the invention, 2,2-diethyl acetoacetate is adopted as a raw material. Bisbenzylbiotin dimer is not generated from obtained bisbenzylbiotin diethyl ester. The reaction yield is high, and the yield of d-biotin is high.
Owner:ZHEJIANG NHU CO LTD
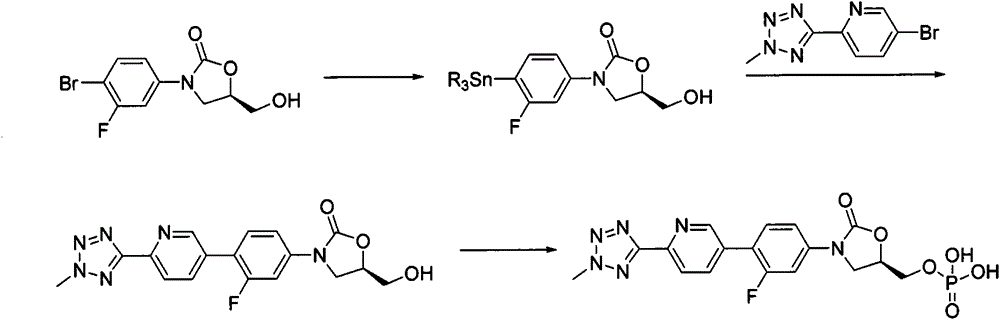
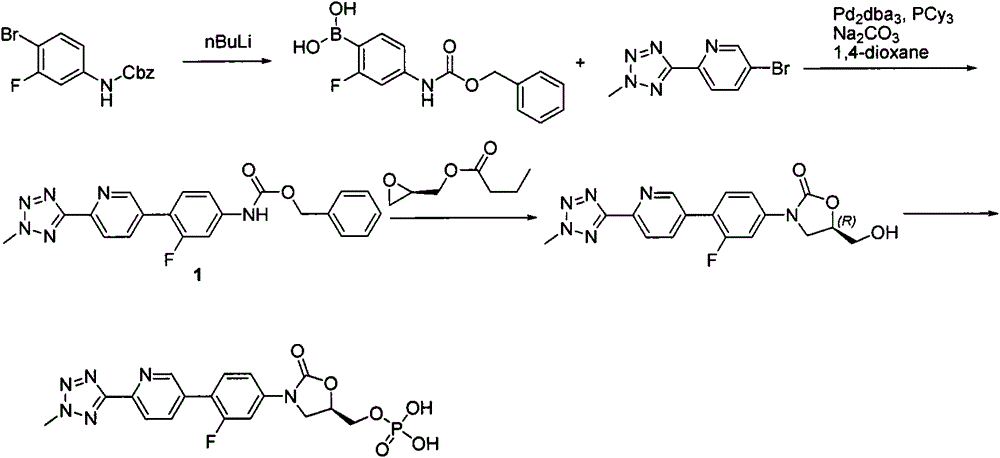
New synthesis process of oxazolinone antibiotic
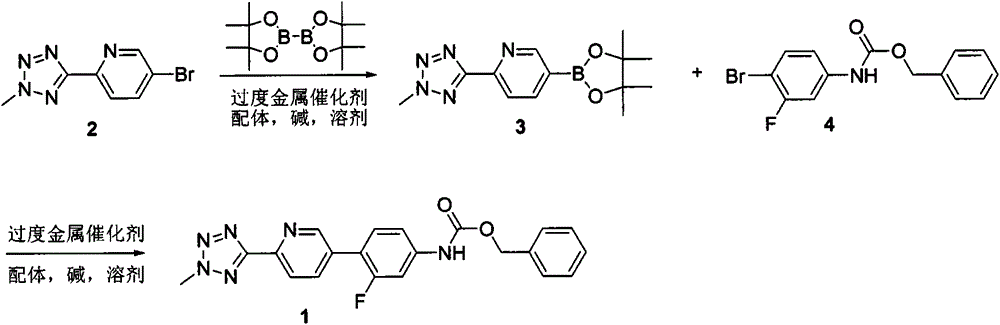
According to the method of the present invention, a methyl tetrazole pyridine bromide (2) and pinacol diboron are subjected to a reaction under catalysis of a transition metal to obtain a boronic acid pinacol ester (3), the compound (3) is separated or is not separated, and the separated compound (3) or the un-separated compound (3) and Cbz-protected bromobenzene (4) are subjected to a reaction under catalysis of a transition metal to obtain a key intermediate (1) of tedizolid. According to the present invention, the compound (3) is not subjected to separation purification, and reacts with the compound (4) in a kettle to generate the compound (1).
Owner:BEIJING CHEMPION BIOTECHNOLOGY CO LTD
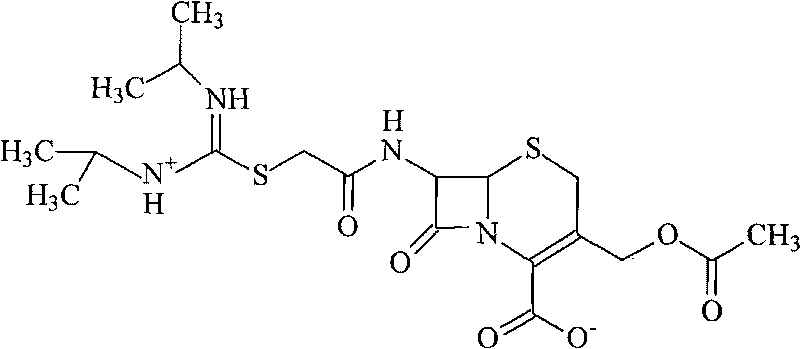
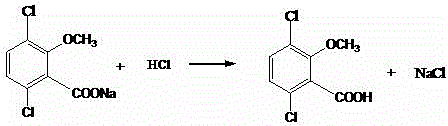
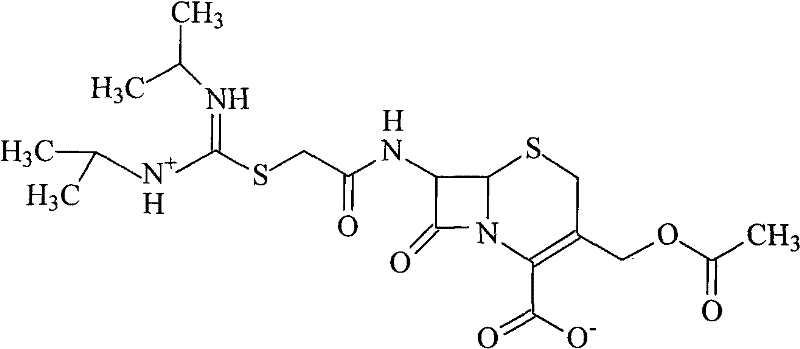
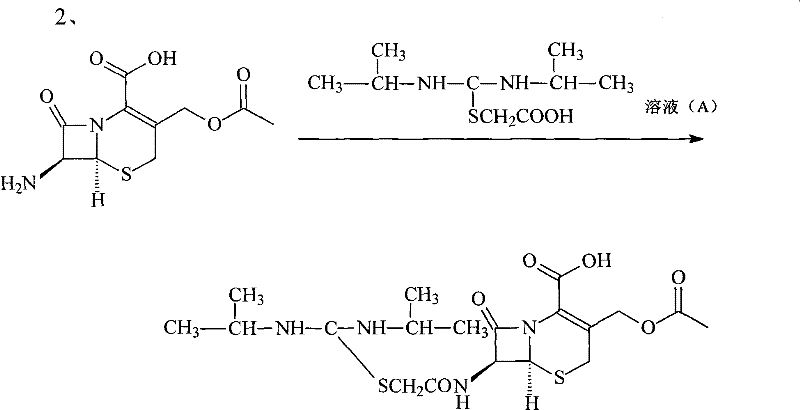
Novel route for cefathiamidine compounds
InactiveCN101704827AMeet the requirementsLower reaction yieldAntibacterial agentsOrganic chemistrySodium bicarbonateThiourea
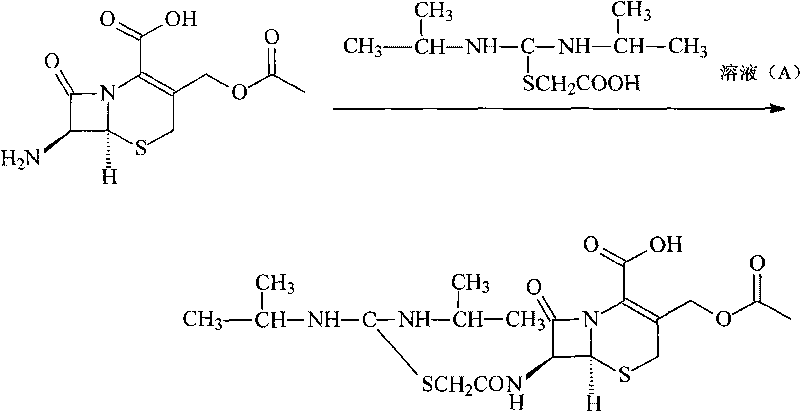
The invention provides a novel route for cefathiamidine compounds. The new route comprises the following steps of: reacting N,N'-diisopropyl thiourea and sodium bicarbonate, adding 2-bromacetic acid in the mixture for reaction, adding triphosgene and triphenylphosphine oxide serving as catalysts, reacting the product of the previous reaction with N,N'-diisopropyl amidine sulfur radical acetic acid to obtain solution(A), and adding the solution(A) to aqueous solution of 7-ACA dropwise for reaction to obtain the target product. The cefathiamidine compounds prepared by the method have the advantages of higher purity and yield, low-priced raw materials, simple synthesis process, simple equipment and easy separation and purification of products.
Owner:HAINAN LINGKANG PHARMA CO LTD
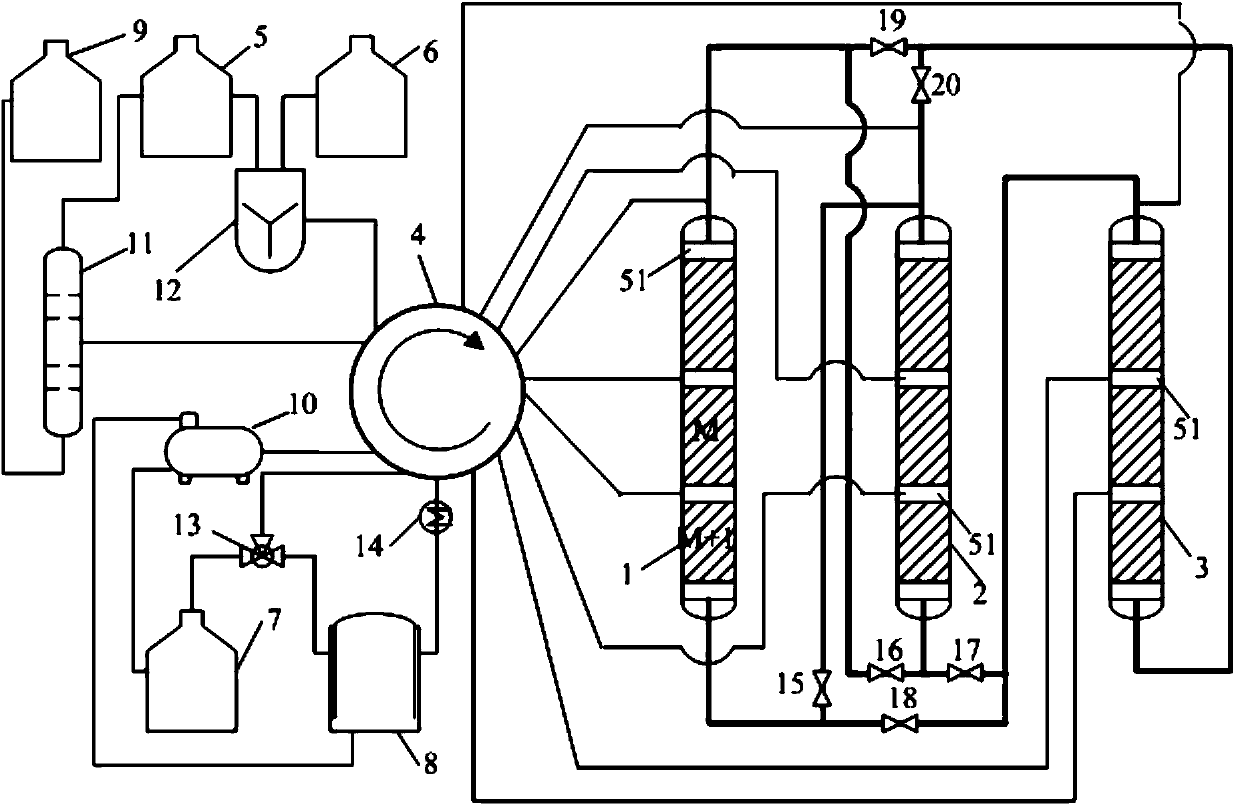
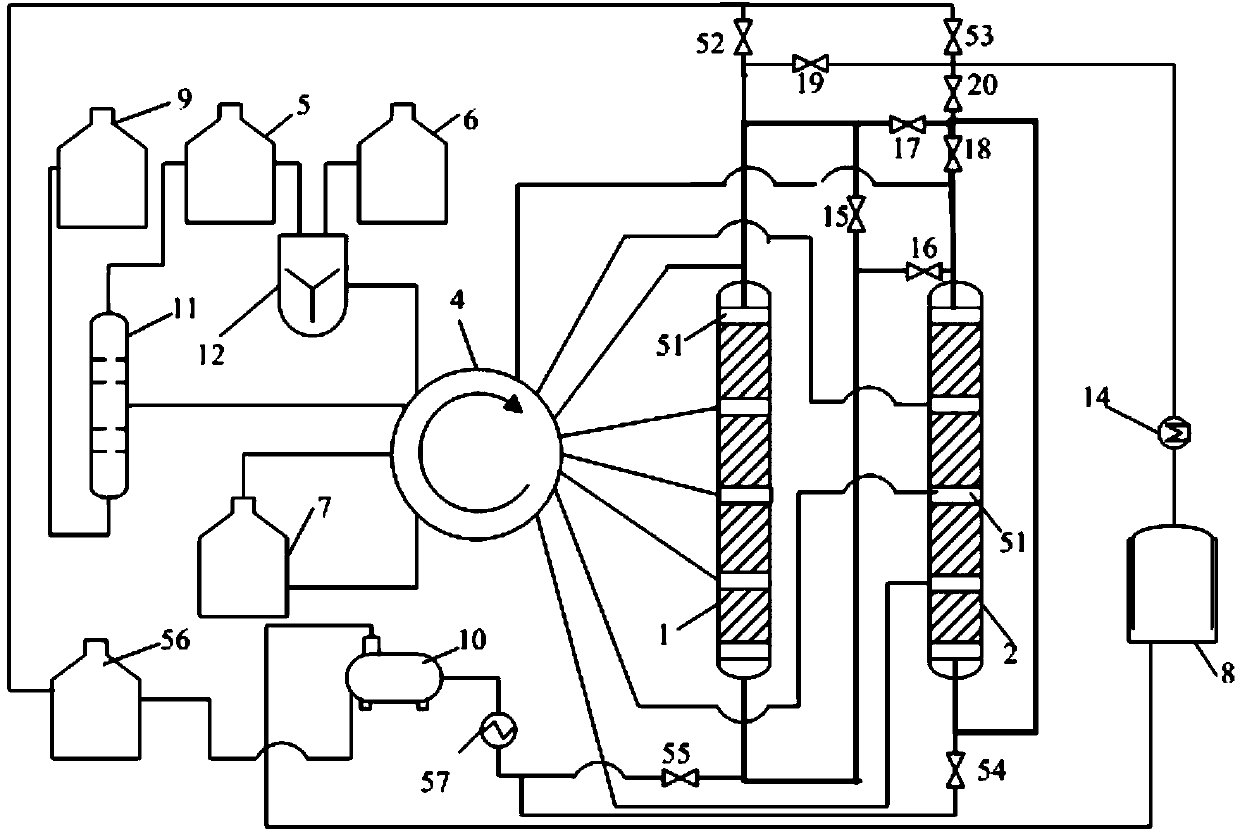
Simulated moving bed reaction and regeneration device for solid acid alkylation and raw material reaction and catalyst regeneration method
ActiveCN105567305ASimple structureFully contactedLiquid hydrocarbon mixtures productionCatalyst regeneration/reactivationAlkyl transferSimulated moving bed
The invention discloses a simulated moving bed reaction and regeneration device for solid acid alkylation and a raw material reaction and catalyst regeneration method. The device comprises a raw material and product zone, a regeneration material zone, a rotary valve and a counter-current simulated moving bed reactor. Under control of the rotary valve, a raw material in the raw material and product zone is introduced into the counter-current simulated moving bed reactor for an alkylation reaction, a product produced in the counter-current simulated moving bed reactor returns to the raw material and product zone, a catalyst regeneration medium in the regeneration material zone is introduced into the counter-current simulated moving bed reactor for catalyst regeneration, and a material produced after catalyst regeneration in the counter-current simulated moving bed reactor returns to the material regeneration zone. By means of the device and the method, the continuous and stable operation of the solid acid alkylation reaction, product extraction, moderate catalyst generation and deep catalyst generation is achieved, the operation cycle and the service life of the device are prolonged, and the operation economy of the device is greatly improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
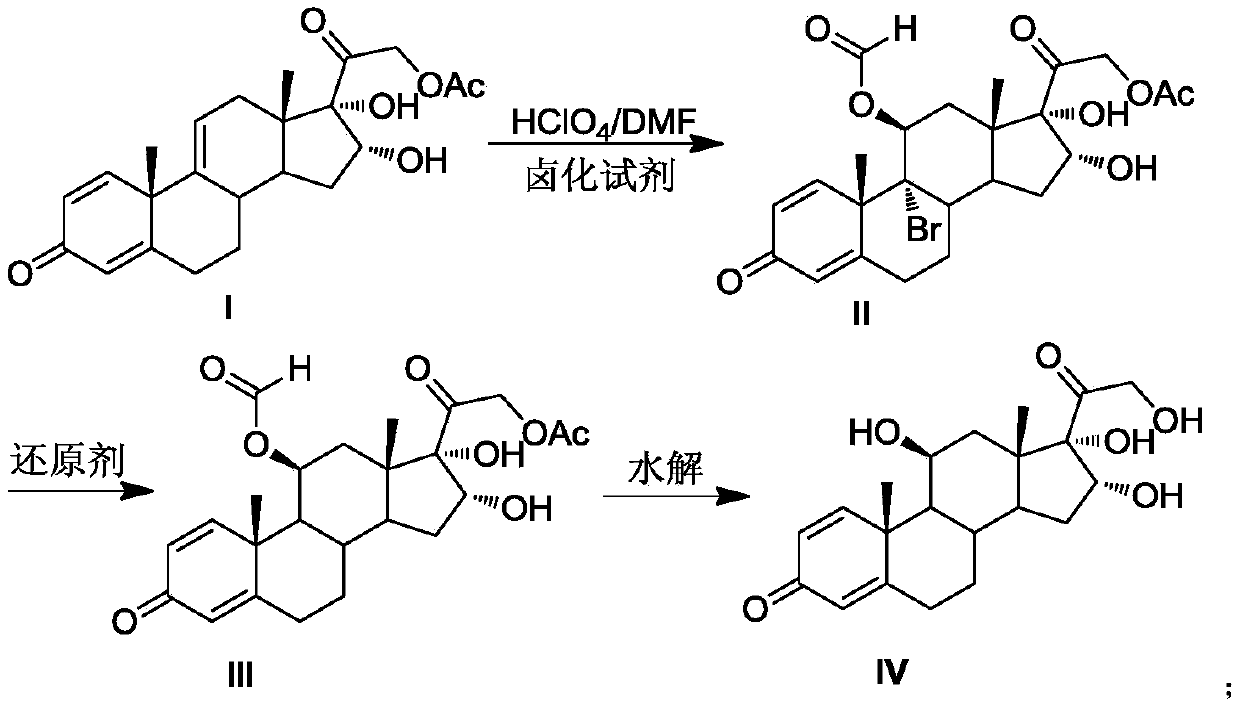
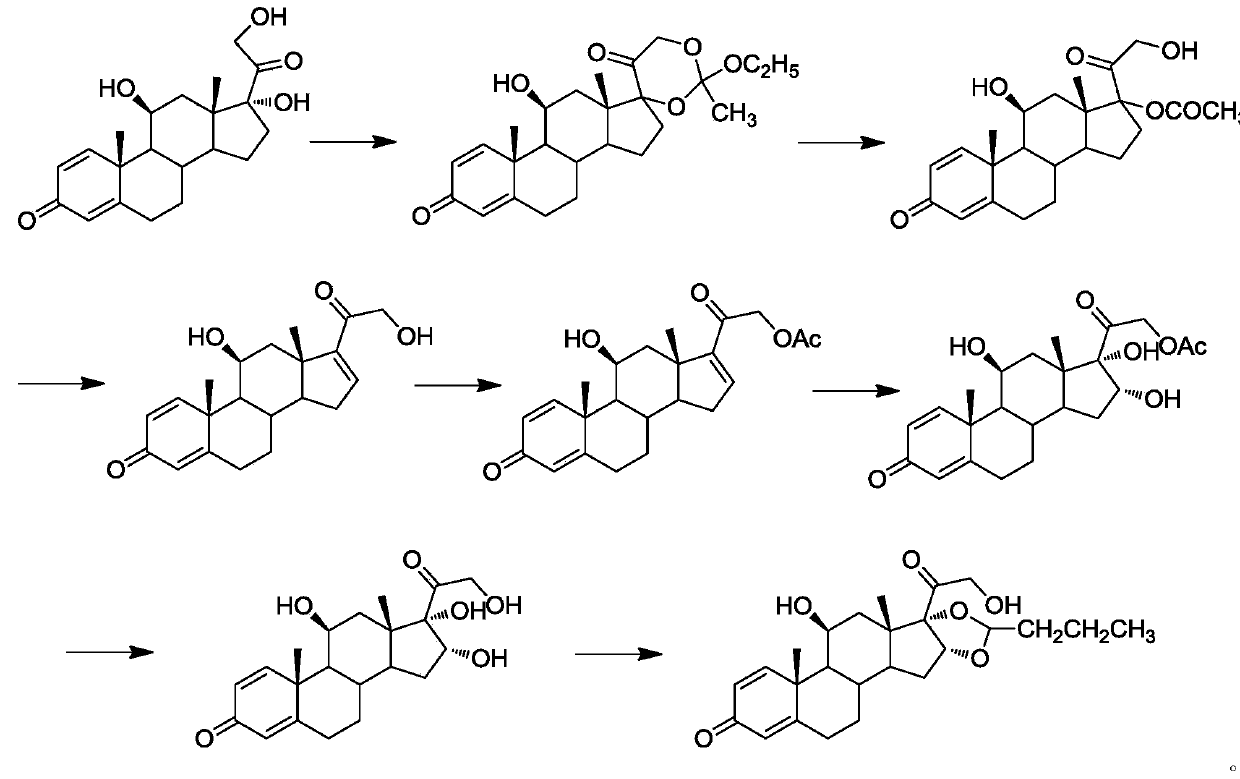
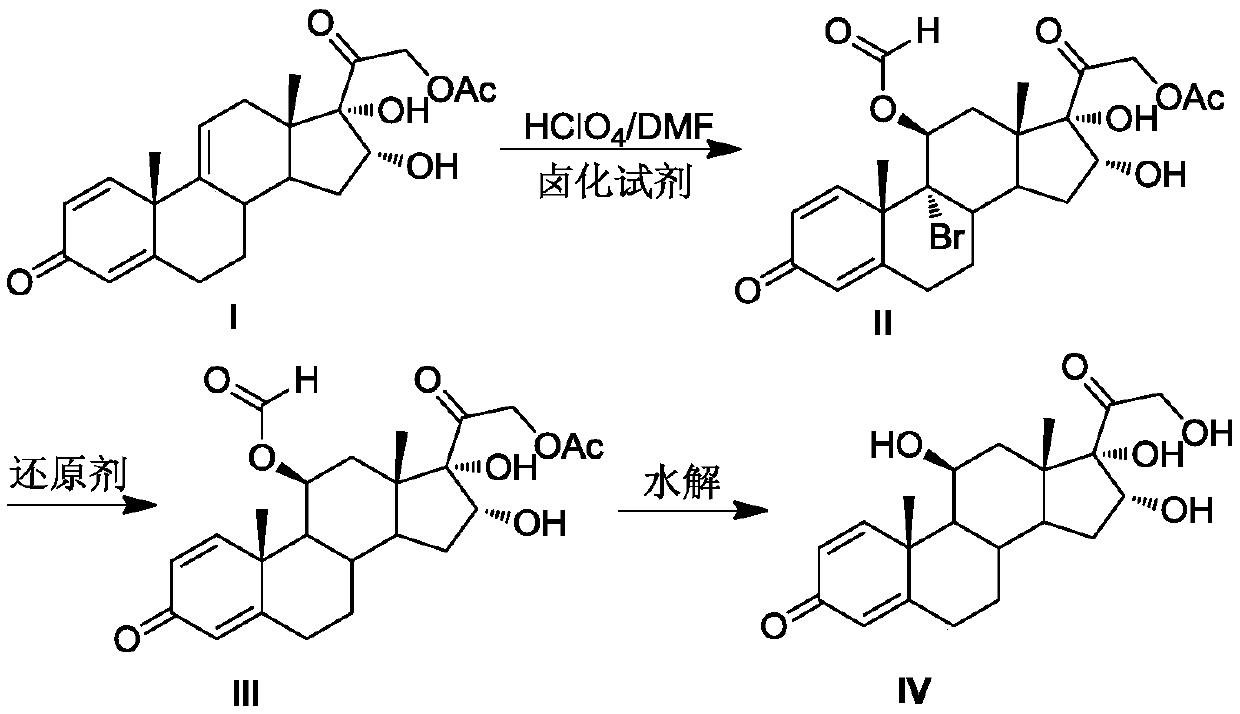
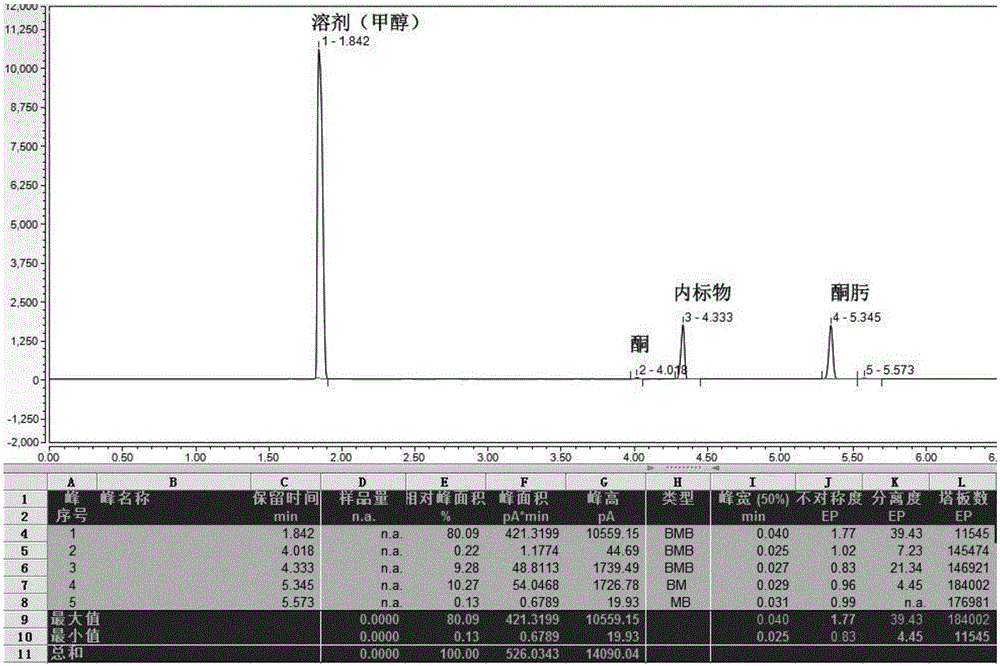
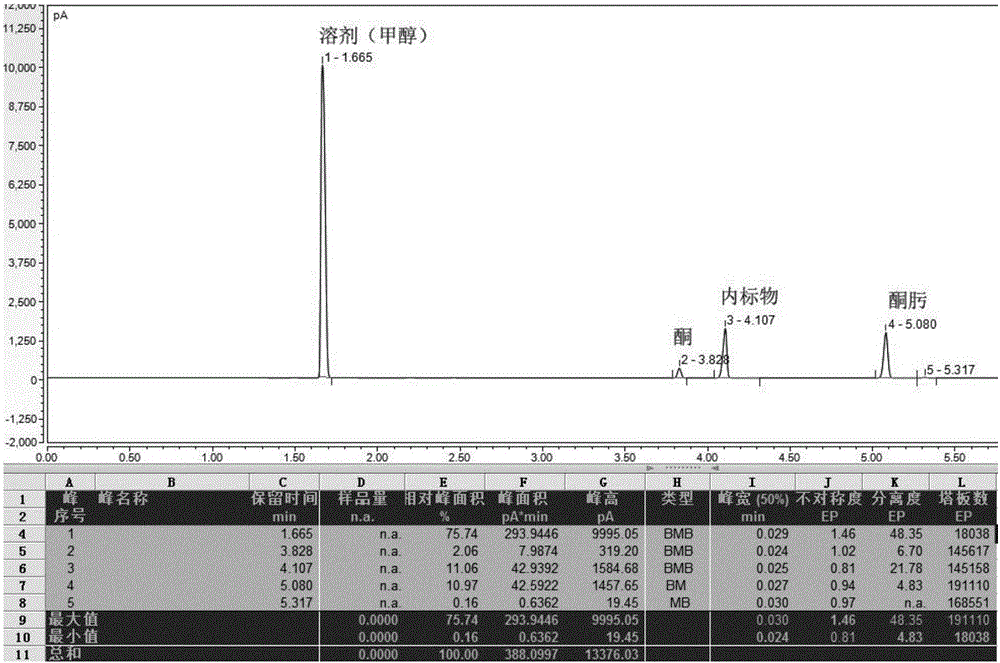
Preparation method of 16alpha-hydroxyprednisolone
The invention discloses a preparation method of 16alpha-hydroxyprednisolone. The method comprises the following steps: dissolving a 16,17,21-trihydroxypregn-1,4,9(11)-triene-3,20-dione-21-acetate compound I and a halogenating reagent into N,N-dimethylformamide, adding HClO4 to form a 11,16,17,21-tetrahydroxypregn-1,4-diene-9-bromo(chloro / iodo)-3,20-dione-11-formate-21-acetate compound 11; adding adehalogenation reagent and a hydrogen donor to remove a 9-position halogen; and finally adding an alkaline liquid, performing a hydrolysis reaction, and adding an acid for neutralization to obtain the 16alpha-hydroxyprednisolone. The method provided by the invention achieves remarkable effects, has the advantages of fewer reaction steps, a high conversion rate, less impurities, low costs and simple operation, is suitable for industrialized production, and has greater application value.
Owner:AURISCO PHARMACEUTICAL CO LTD
Technological method for synthesizing ketoxime
ActiveCN106831486AImprove conversion rateQuick responseOximes preparationMolecular sieveReaction rate
The invention discloses a technological method for synthesizing ketoxime. In a system of a carbonate water solution, a titanium silicalite molecular sieve is adopted as a catalyst, and ketone, ammonia and hydrogen peroxide react to generate ketoxime; the pH value of the reaction system is monitored in real time in the reaction process to judge the reaction process and determine the optimal reaction ratio. The pH value in the reaction system is monitored to judge the reaction process and determine the optimal reaction ratio, and then the pH value of the system is adjusted through a carbonate water solution in order to increase the reaction rate and the conversion rate of ammonia.
Owner:CHEM TECH ACAD OF SHANDONG PROVINCE
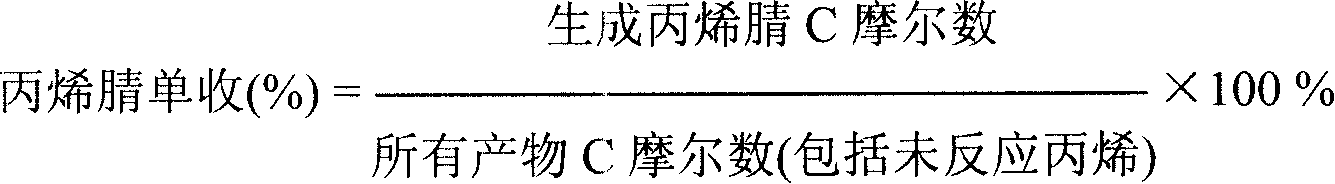
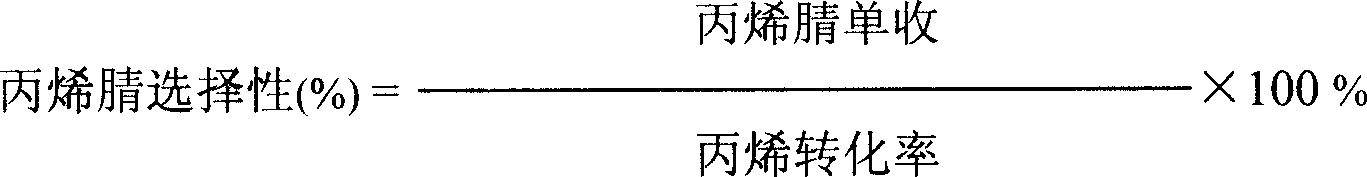
Catalyst for preparing acrylonitrile by ammonia oxidizing method
ActiveCN101147869ASuppress generationHigh yieldPreparation by hydrocarbon ammoxidationMetal/metal-oxides/metal-hydroxide catalystsChemistryNH3 compound
The present invention relates to a fluidized bed catalyst for producing acrylonitrile by using ammonia oxidation process. It is characterized by that said catalyst uses silicone dioxide or aluminum oxide or their mixture as carrier, and contains the following active components: Mo, Bi, Fe, Ni, X, Y, Z and Q, in which X is at least one kind selected from Mg, Co, Ca, Be, Cu, Zn, Pb, Mn or Te, Y is at least one kind selected from La, Ce or Sm, Z is at least one kidn selected from K, Rb, Na, Li o Cs and Q is at least one kidn selected from Ti, Zr, Nb or Sb. Said catalyst can be used in industrial production of acrylonitrile by using propene ammonia oxidation process.
Owner:CHINA PETROLEUM & CHEM CORP +1
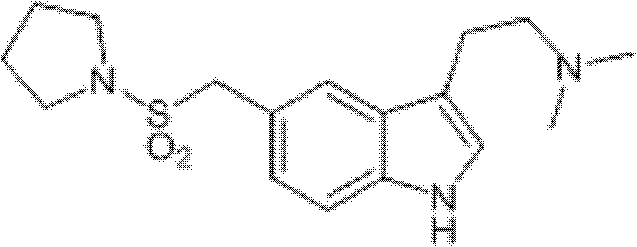
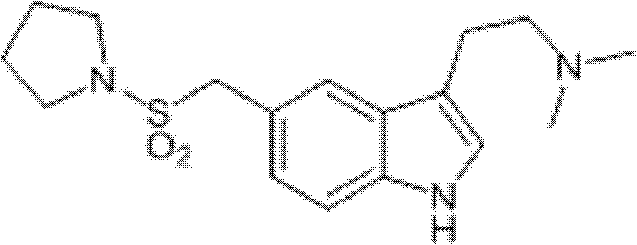
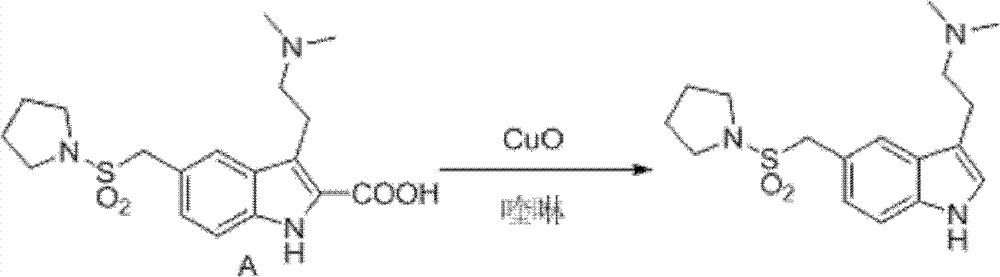
Method for preparing migraine resistant medicine Almotriptan
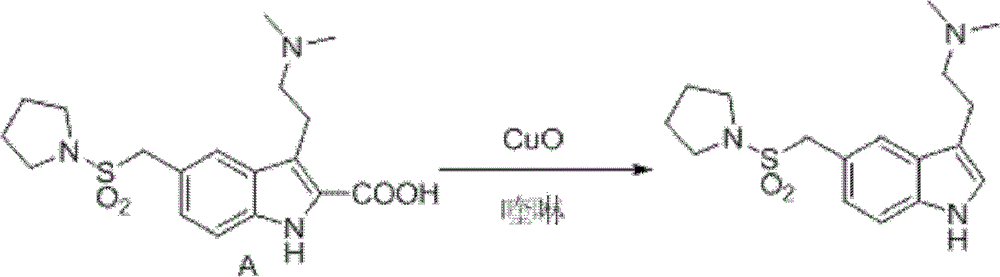
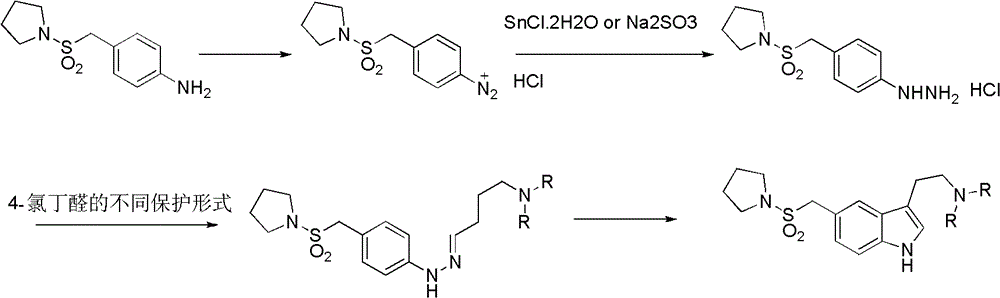
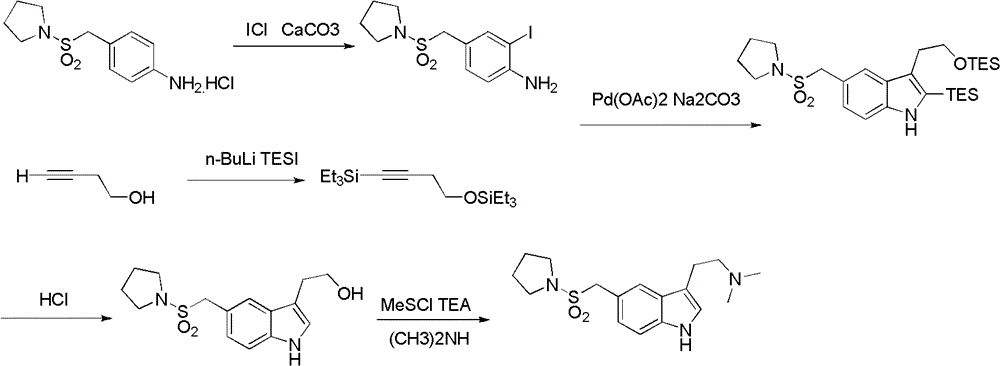
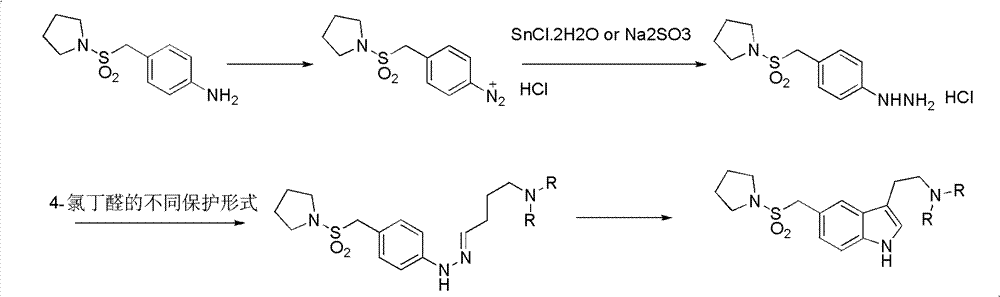
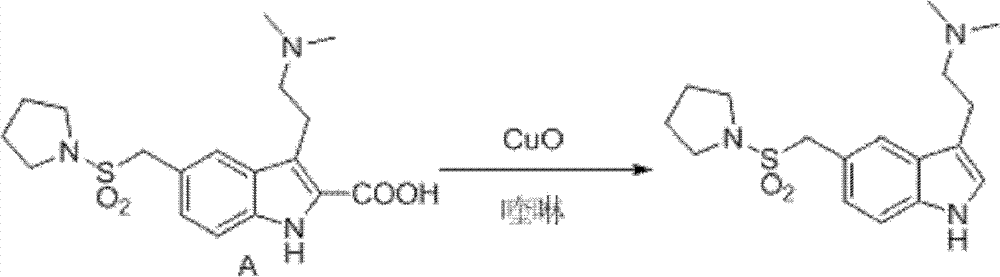
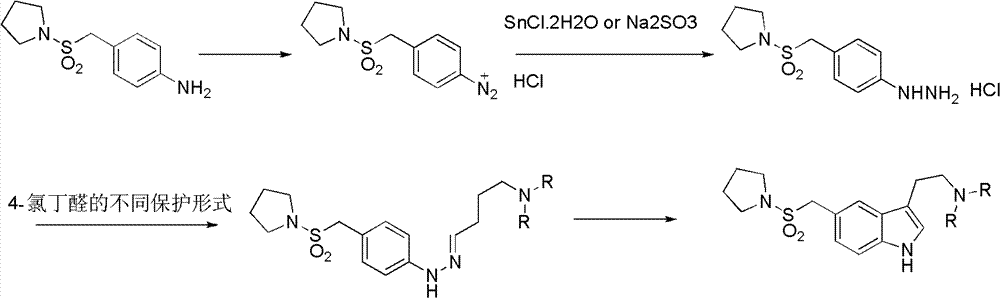
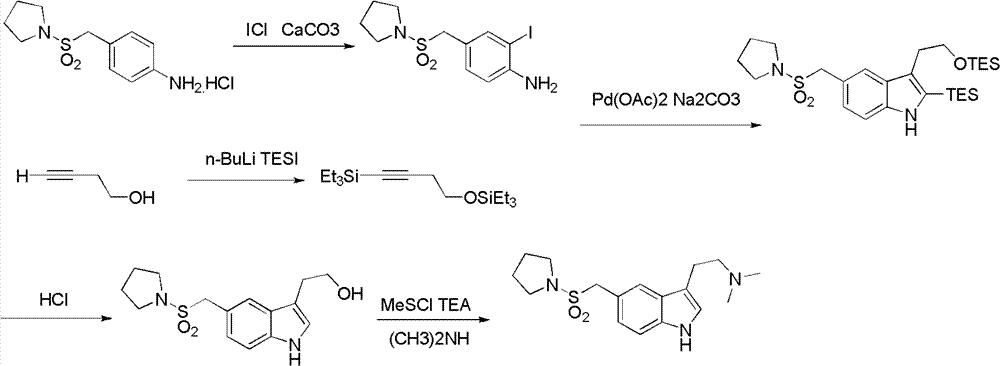
InactiveCN102775391ALow purityLower reaction yieldGroup 4/14 element organic compoundsNervous disorderAlmotriptanAntimigraine Agents
The invention provides a method for preparing a migraine resistant medicine Almotriptan. The method comprises the following steps: respectively or simultaneously removing R1 and R2 from an N-protected-3,5-disubstituted indole derivative which is adopted as an initial raw material, and then collecting Almotriptan from a reaction system. The method has the advantages of safety, mild reaction conditions, easy operation, high yield, cheap and easily obtained raw material, easy treatment of three wastes, and convenient industrialized production. The chemical formula of the N-protected-3,5-disubstituted indole derivative is represented by formula (I) in the specification.
Owner:SHANGHAI INST OF PHARMA IND
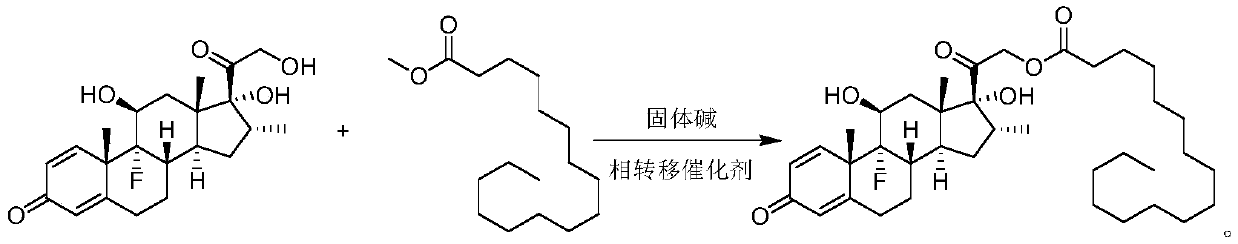
Synthesis method of dexamethasone palmitate
The invention relates to a synthesis method of dexamethasone palmitate. The method comprises the steps as follows: dexamethasone and methyl palmitate are subjected to transesterification under catalysis of solid alkali and a phase transfer catalyst to obtain a target product; a mixture of methyl palmitate and the product obtained after a solvent is removed from solid alkali and the phase transfercatalyst by evaporation is recycled. The method is mild in reaction condition, green, environmentally friendly, simple, efficient and energy-saving, raw materials are easy to obtain, the production cost is reduced, the operation environment is improved, and the method is convenient to operate, high in automation degree and particularly prone to industrialization.
Owner:JIANGSU YUANDA XIANLE PHARMA
Ammoxidation method to manufacturing unsaturated nitrile fluid-bed catalyst
ActiveCN101121131ASuppress generationHigh yieldPreparation by hydrocarbon ammoxidationMetal/metal-oxides/metal-hydroxide catalystsFluidized bedActive component
The present invention relates to a fluidized bed catalyst of unsaturated nitrile which is produced by an ammoxidation. The present invention mainly solves the problems in the prior art that a yield of an acrylonitrile catalyst in the reaction of the industrial device is not high, and the yield is gradually reduced with the prolonged time; the by-product s of CO2, CO and HCN are increased; the stability of the catalyst is not good enough. The present invention solves the problems well by a technical proposal that a catalyst carrier is selected from SiO2 or Al2O3a or a mixture of SiO2 and Al2O3, and the catalyst contains active components, the general formula of which is as follows: in the formula of Mo12BiaFebNicXdYeZfQgOx, the X is selected from at least one of Mg, Co, Ca, Be, Cu, Zn, Pb, Mn or Te; the Y is selected from at least one of La, Ce or Sm; the Z is selected from at least one of K, Rb, Na, Li or Cs; the Q is selected from at least one of Ga, Ge or Pr. The present invention can be used for the industrial production of acrylonitrile which is produced by a propylene ammoxidation.
Owner:CHINA PETROLEUM & CHEM CORP +1
Furfural preparation process
PendingCN111848557ALower reaction yieldEnhanced dehydration selectivityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsProcess engineeringAldehyde
The invention discloses a furfural preparation process. The furfural preparation method adopts a two-step method. The method comprises the following specific steps: firstly, catalyzing hydrolysis of hemicellulose in corncobs by adopting an SO4 < 2-> / Fe2O3-alpha-Al2O3 supported solid acid catalyst; then catalyzing corncob hydrolysis reaction liquid to prepare furfural by using a novel catalyst instead of sulfuric acid, stripping crude aldehyde in the reaction system by using nitrogen instead of water vapor, performing alkali washing and refining on the stripped crude aldehyde by using a continuous centrifugal extraction refining method, introducing the crude aldehyde into a rectifying tower, and rectifying to obtain furfural with the purity of over 99%. According to the method, the furfuralis prepared through the two-step method, lignin and cellulose in the corncob residues can be reserved for reuse, the concentration of the crude aldehyde is increased through nitrogen steam stripping,the furfural production yield is increased, and the wastewater yield is reduced.
Owner:QINGDAO UNIV OF SCI & TECH
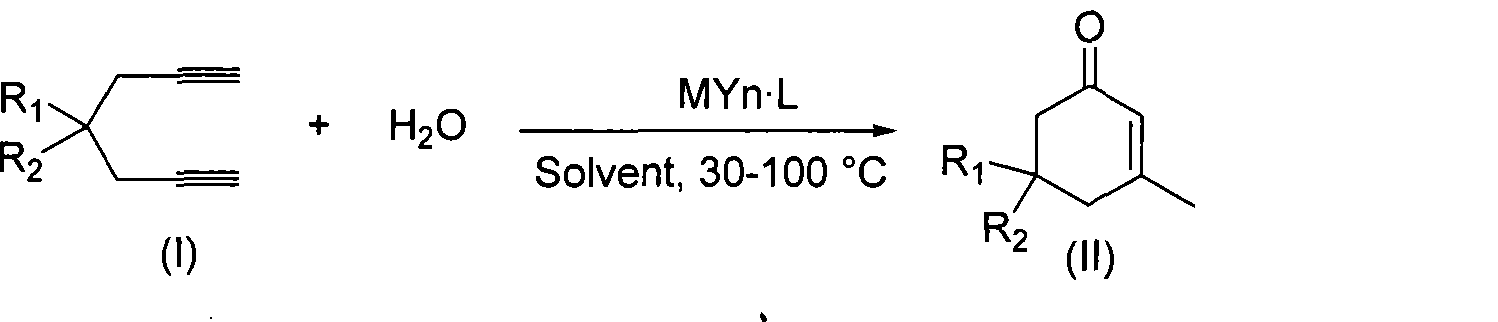
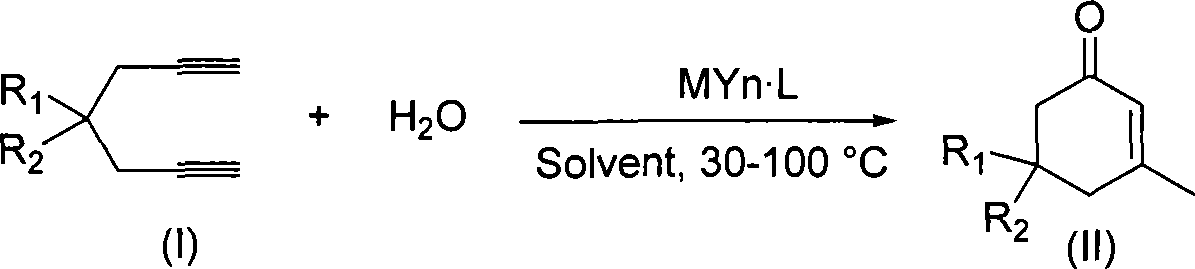
Preparation of 2-pimelie kelone compound
ActiveCN101412667AEasy to operatePromote reaction efficiencyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCyclohexenoneChemical products
The invention discloses a method for preparing 2-cyclohexenone compound, which comprises the following steps: under the action of a metallic catalyst MYn.L, 1, 6-heptadiyne compound showed in formula (I) taken as a raw material is reacted in an ionic liquid at a temperature of between 0 and 150 DEG C; after the reaction liquid is treated, the 2-cyclohexenone compound showed in formula (II) is prepared; and at the same time, the ionic liquid fixed with the metallic catalyst can be recycled. The method has the advantages of simple and reliable operation, high yield and selectivity, repeated recycling and use of the noble metal catalyst, environmental protection, and the like. Because the cyclohexenone compound is a good raw material for synthesizing medicines, pesticides and chemical products, the method has wide industrial application prospect.
Owner:ZHEJIANG UNIV OF TECH
Process of preparing dicamba by 3, 6-dichlorosalicylic acid
InactiveCN105906503AHigh yieldIncrease profitPreparation from carboxylic acid saltsOrganic compound preparationWastewaterSaponification
The invention relates to a process of preparing dicamba by 3, 6-dichlorosalicylic acid, and belongs to the technical field of herbicide dicamba preparation processes. The process includes the steps of methylation reaction, saponification, methanol recovery, acidizing reaction and the like. By means of saponification with specific process parameters, efficiency can be greatly improved, saponification time is shortened, wastewater quantity is remarkably reduced, and equipment utilization rate is increased.
Owner:SICHUAN FOURSTAR BIOTECH RANDD CORP
Synthesis method of cefathiamidine compound
InactiveCN101704827BMeet the requirementsLower reaction yieldAntibacterial agentsOrganic chemistrySodium bicarbonateSynthesis methods
Owner:HAINAN LINGKANG PHARMA CO LTD
Preparation device and preparation method of nicarbazin
ActiveCN106496131AHigh yieldEasy to handleUrea derivatives preparationOrganic compound preparationP-NitroanilineBottle
The invention provides a preparation method of nicarbazin. The preparation method includes the following steps: 1, adding wet paranitroaniline and a solvent into a reaction bottle, and conducting heating, refluxing and water separation; 2, after conducting water distribution, conducting cooling to the room temperature, adding bis(trichloromethyl)carbonate, then conducting heating and stirring to obtain 4, 4'-binitro diphenylcarbamide, and absorbing the generated hydrogen chloride gas with the solvent; 3, adding the liquid absorbing the hydrogen chloride gas as a reaction solvent in another reaction bottle, and adding acetylacetone and urea for a reaction to generate 2-hydroxyl-4,6-dimethyl pyrimidine hydrochloride; 4, regulating the obtained 2-hydroxyl-4,6-dimethyl pyrimidine hydrochloride to be alkaline, then allowing 2-hydroxyl-4,6-dimethyl pyridine hydrochloride to react with 4,4'-binitro diphenylcarbamide in a solution to generate the nicarbazin under the stirring condition, and conducting processing to obtain the finished product of the nicarbazin. The special devices are used and comprise a reaction kettle, a condenser, a gas-liquid separator, a water separator, an anti-inverse-suction one-way valve, an absorption kettle and a second reaction kettle. The raw materials are fully utilized, the product yield is high, the cost is low, and the three wastes are few.
Owner:HUBEI ZHONGMU ANDA PHARMACEUTICAL CO LTD +1
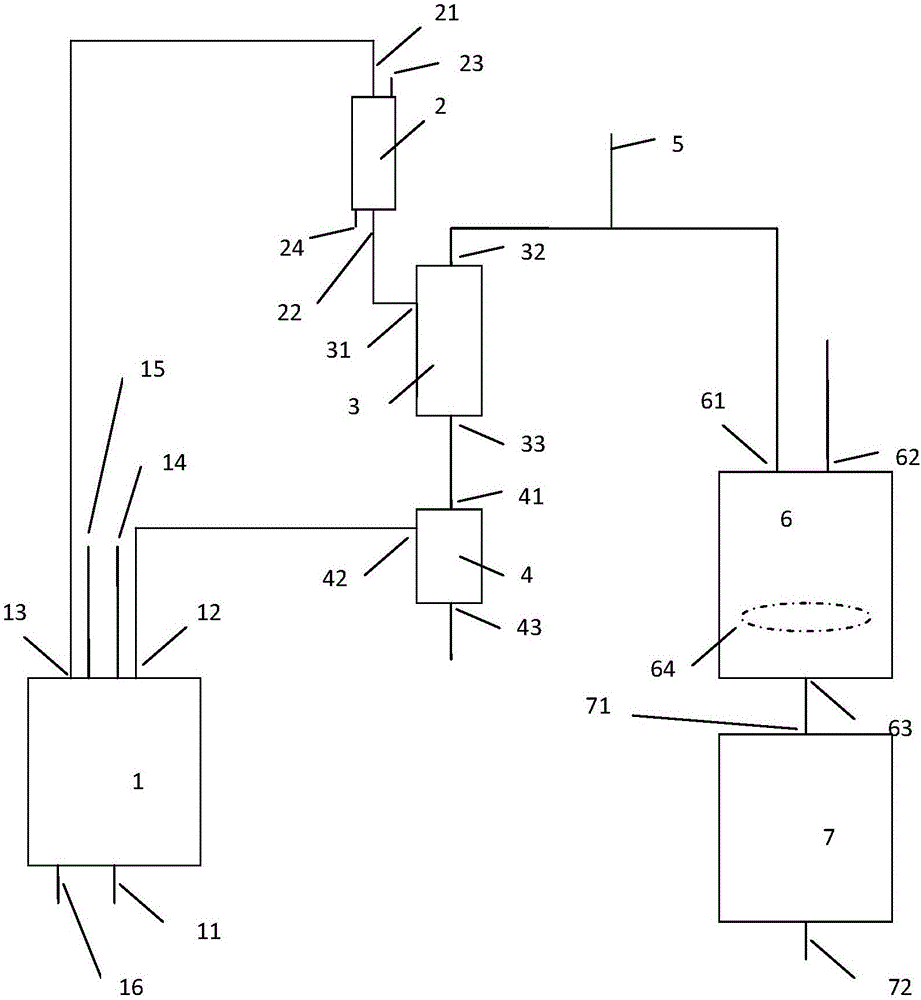
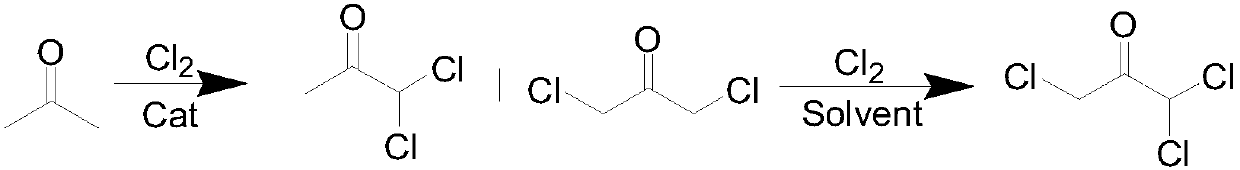
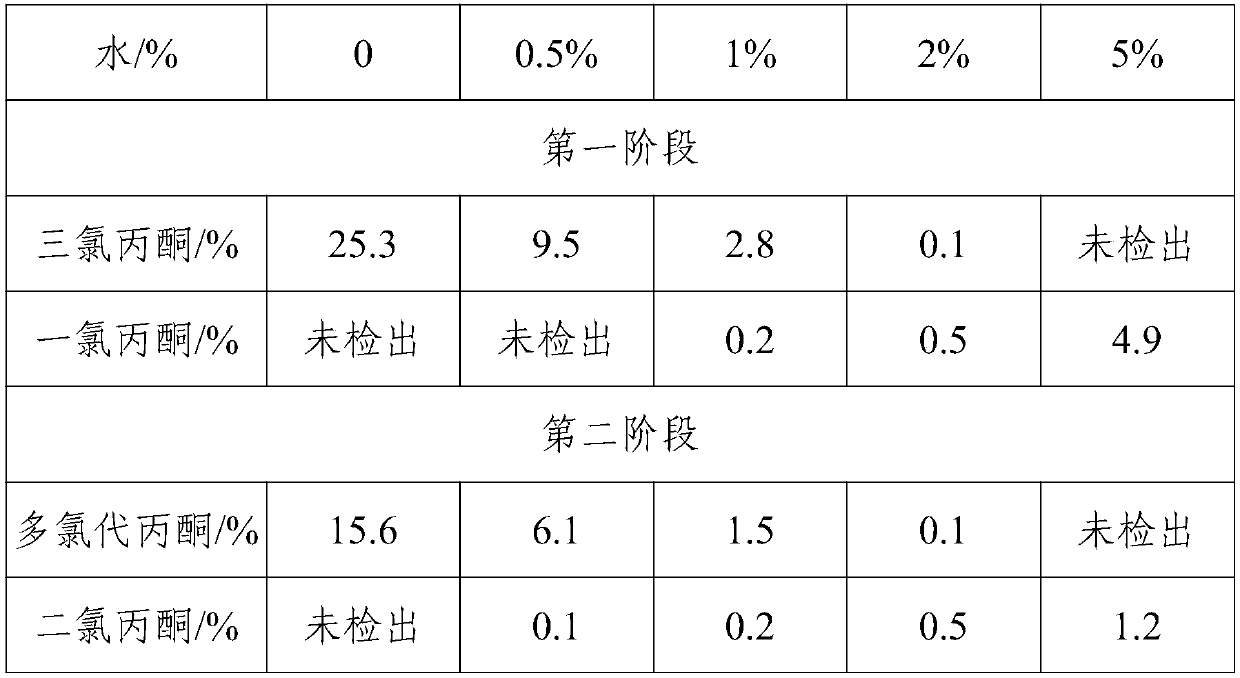
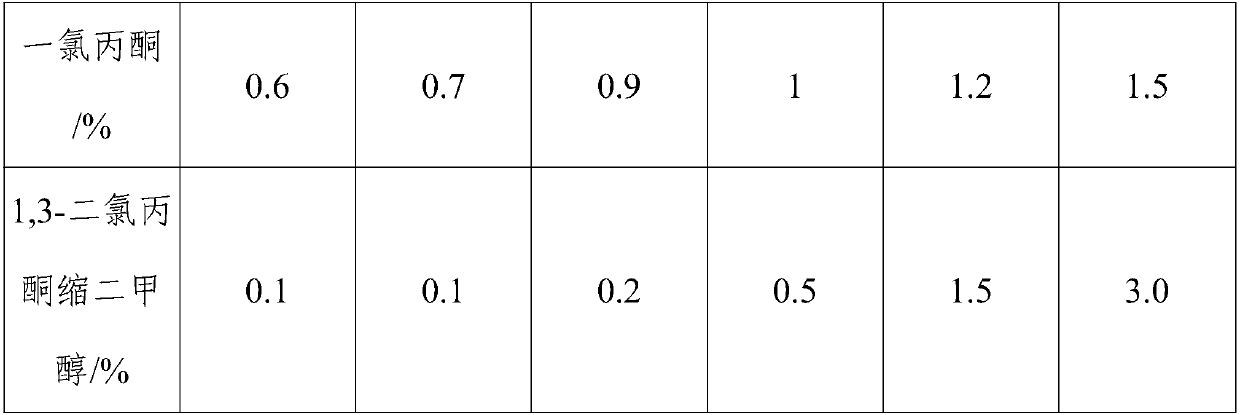
Method for preparing 1,1,3-trichloroacetone through high-selectivity chlorination of acetone
ActiveCN107602364AAvoid generatingCan control the reaction processOrganic compound preparationCarbonyl compound preparationSolventFolic acid
Owner:ANHUI COSTAR BIOCHEM CO LTD
Preparation method of important intermediate of novel febuxostat
InactiveCN101759657AWide variety of sourcesOvercoming harmful flawsOrganic chemistryXanthine oxidase inhibitorEthyl ester
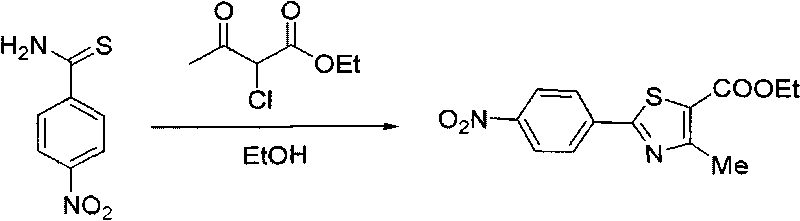
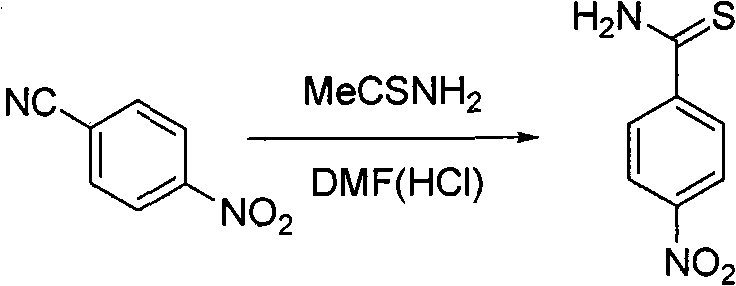
The invention relates to a preparation method of important intermediate 2-[3-cyan-(2-isobutoxy) phenyl]-4-methyl-5-thiazolecarboxylate of febuxostat which is a new generation of xanthine oxidase inhibitor. The method takes 2-(4-nitrobenzophenone)-4-methyl-5-thiazolecarboxylate as material to have cyanation reaction and isobutylation reaction under the existence of dimethyl sulfoxide to produce 2-[3-cyan-(2-methylpropoxy) phenyl]-4-methyl-5-thiazolecarboxylate. The material source is wide, the intermediate is easy to prepare, the reaction condition is mild, the operation is easy and the production cost is low, therefore, the preparation method is suitable for industrial production.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Synthesis method of 2,2-dibromo-2-malonamidenitrile
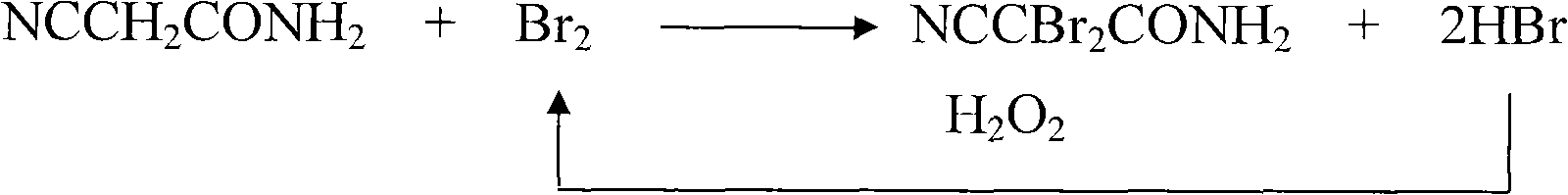
InactiveCN101781233AAvoid decompositionHigh reaction yieldCarboxylic acid nitrile preparationOrganic compound preparationBromineSynthesis methods
The invention discloses a synthesis method of 2,2-dibromo-2-malonamidenitrile. The synthesis method comprises the following steps of: adding malonamidenitrile and a solvent into a reaction bottle, wherein the malonamidenitrile is used as raw materials; dropwise adding liquid bromine while stirring at the reaction temperature of 15-45 DEG C, when the dropwise adding dosage of the malonamidenitrileaccounts for one third to four fifths of a total adding amount, beginning to dropwise adding hydrogen peroxide with the concentration of 30 percent, after dropwise adding the liquid bromine and the hydrogen peroxide, continuing reacting for 0.5-6 hours, and then filtering to obtain the 2,2-dibromo-2-malonamidenitrile, wherein the molar ratio of the malonamidenitrile to the bromine to the hydrogenperoxide is 1:(1.1-1.3):(0.70-0.90), the solvent is water or filtrate, and the dosage of the solvent is 252-420mL malonamidenitrile per mole. The invention is mainly used for the synthesis of the 2,2-dibromo-2-malonamidenitrile.
Owner:XIAN MODERN CHEM RES INST
Tetrabutyl tin preparation method
The invention discloses a tetrabutyl tin preparation method. The preparation method comprises the following steps: adding magnesium powder and an ether solvent into a reactor with a stirring and refluxing device, starting to stir and heat till refluxing; then adding a small quantity of chlorobutane and a YF-017 catalyst, slowly adding a mixed solution of chlorobutane and anhydrous tin tetrachloride dropwise, conducting a refluxing reaction to the end after finishing adding; cooling to the normal temperature, adding hydrochloric acid while stirring, then allowing standing still for layering; releasing an aqueous phase on the lower layer, distilling an organic phase at the normal pressure, changing to perform reduced pressure distillation after the solvent is completely distilled, and collecting a fraction of 148-150 DEG C under the condition of the vacuum degree of minus 650 Pa, to obtain tetrabutyl tin. A small quantity of catalyst is added into a one-step reaction device, the Grignardreaction is started immediately, the heat produced in the Grignard reaction in the first step is rapidly absorbed by the alkylation reaction in the second step. Therefore, the phenomenon of materialspraying of the reaction is avoided, and safety in the industrial production is guaranteed.
Owner:SHANGHAI NO 4 REAGENT & H V CHEM
N-protected-3,5-disubstituted indole derivative and its preparation method and application
InactiveCN102775392BLow purityLower reaction yieldGroup 4/14 element organic compoundsAlmotriptanAntimigraine Agents
The invention provides an N-protection-3, 5-disubstituted indole derivative, its preparation method and application. The N-protection-3, 5-disubstituted indole derivative can be used for preparing the anti-migraine drug Almotriptan. Compared with reported literature, the preparation method provided in the invention has the advantages of: cheap and easily available raw materials, mild reaction conditions, simple operation, greatly shortened reaction steps, quality controllable and high purity product, less pollution of ''three wastes'', and easy industrial production. The N-protection-3, 5-disubstituted indole derivative is a free alkali or salt of the compound having a structural general formula (I).
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Preparation of 2-pimelie kelone compound
ActiveCN101412667BEasy to operatePromote reaction efficiencyOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsCyclohexenoneChemical products
The invention discloses a method for preparing 2-cyclohexenone compound, which comprises the following steps: under the action of a metallic catalyst MYn.L, 1, 6-heptadiyne compound showed in formula (I) taken as a raw material is reacted in an ionic liquid at a temperature of between 0 and 150 DEG C; after the reaction liquid is treated, the 2-cyclohexenone compound showed in formula (II) is prepared; and at the same time, the ionic liquid fixed with the metallic catalyst can be recycled. The method has the advantages of simple and reliable operation, high yield and selectivity, repeated recycling and use of the noble metal catalyst, environmental protection, and the like. Because the cyclohexenone compound is a good raw material for synthesizing medicines, pesticides and chemical products, the method has wide industrial application prospect.
Owner:ZHEJIANG UNIV OF TECH
Biodiesel hydrodeoxygenation technique
InactiveCN106244187AHigh deoxygenation rateReduce catalytic activityMolecular sieve catalystsBiofuelsBiodieselHydrogen pressure
The invention discloses a biodiesel hydrodeoxygenation technique. The technique is characterized by adopting a fixed-bed reactor, wherein the fixed-bed reactor is filled with a hydrodeoxygenation catalyst, and the catalyst comprises a support and an active component. The support is MCM-41 doped with heteroatom Cu<2+> in a synthetic skeleton structure. The active component is a mixture of dimolybdenum nitride Mo2N, tungsten nitride W2N, molybdenum carbide Mo2C and tungsten carbide WC. The catalyst also contains a catalytic aid, and the catalytic aid is a mixture of TiO2, CeO2, V2O5 and NbOPO4. The reaction conditions of the fixed-bed reactor are as follows: the reaction temperature is 300-450 DEG C, the hydrogen pressure is 2.5-3.5 MPa, the hydrogen / oil volume ratio is 500-800, and the volumetric space velocity is 1.0-2.5 h<-1>. When the technique is used for biodiesel hydrodeoxygenation, the deoxygenation rate can be up to 99.8% or above, and the catalytic activity after 500-hour continuous operation does not obviously decrease.
Owner:锡山区绿春塑料制品厂
Preparation method of anti-migraine drug Almotriptan
InactiveCN102775342ALow purityLower reaction yieldOrganic chemistryBulk chemical productionAlmotriptanSolvent
The invention provides a preparation method of an anti-migraine drug Almotriptan. The method includes the following steps of: taking an N-protection-3, 5-disubstituted indole derivative as a starting material, removing the N-protection group in a solvent and in the presence of a catalyst, and then collecting Almotriptan from the reaction system. The method provided in the invention for preparing Almotriptan has the advantages of safety, mild reaction condition, easy operation, high yield, cheap and easily available raw materials, easy ''three wastes'' treatment, and convenient industrial implementation. The N-protection-3, 5-disubstituted indole derivative has a chemical structural formula as shown in formula (I).
Owner:SHANGHAI INST OF PHARMA IND CO LTD
N-protection-3, 5-disubstituted indole derivative, its preparation method and application
InactiveCN102775392ALow purityLower reaction yieldGroup 4/14 element organic compoundsAntimigraine AgentsMedicinal chemistry
The invention provides an N-protection-3, 5-disubstituted indole derivative, its preparation method and application. The N-protection-3, 5-disubstituted indole derivative can be used for preparing the anti-migraine drug Almotriptan. Compared with reported literature, the preparation method provided in the invention has the advantages of: cheap and easily available raw materials, mild reaction conditions, simple operation, greatly shortened reaction steps, quality controllable and high purity product, less pollution of ''three wastes'', and easy industrial production. The N-protection-3, 5-disubstituted indole derivative is a free alkali or salt of the compound having a structural general formula (I).
Owner:SHANGHAI INST OF PHARMA IND CO LTD
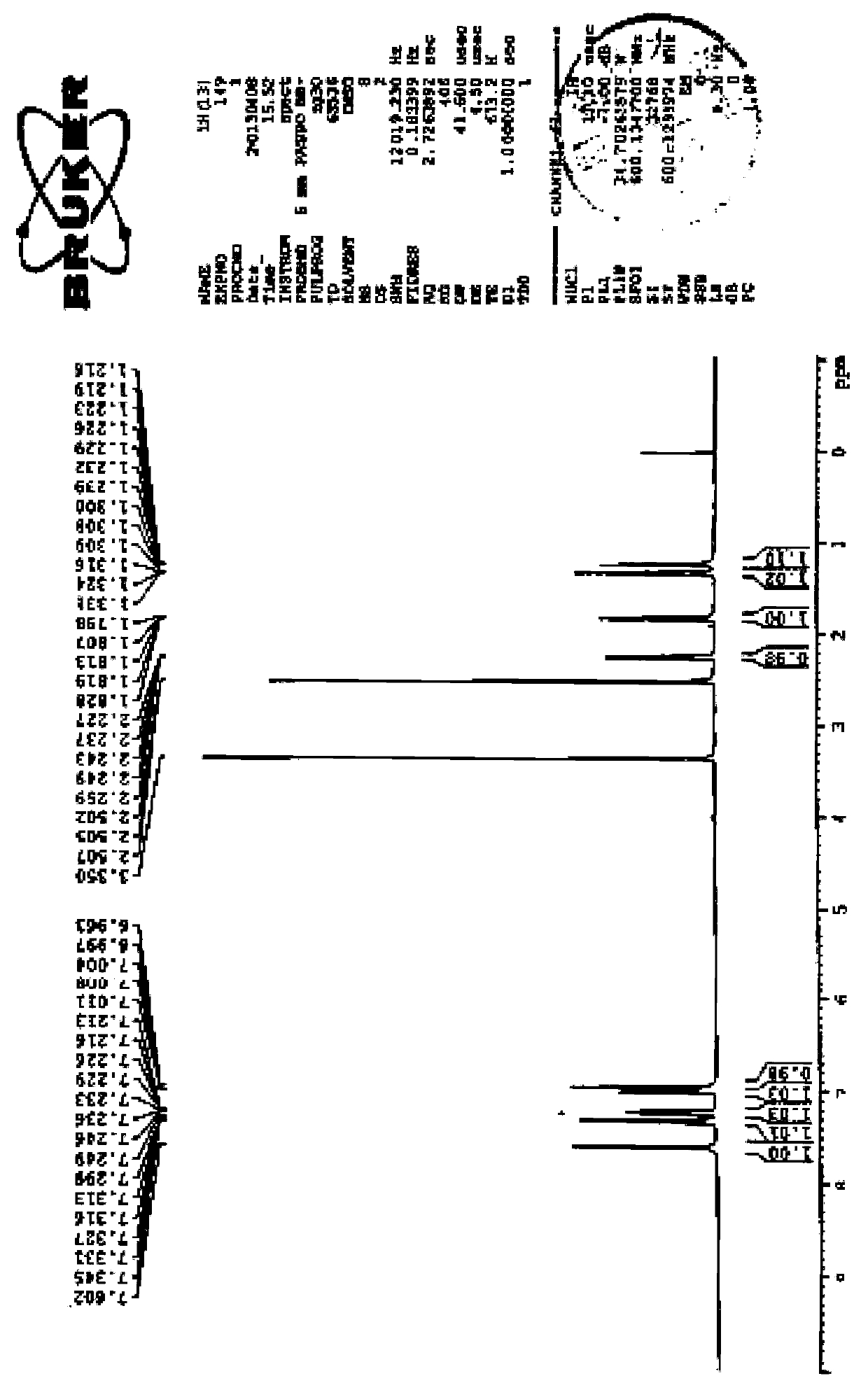
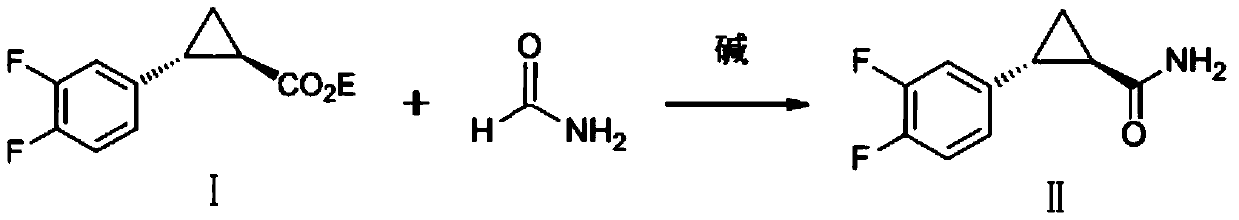
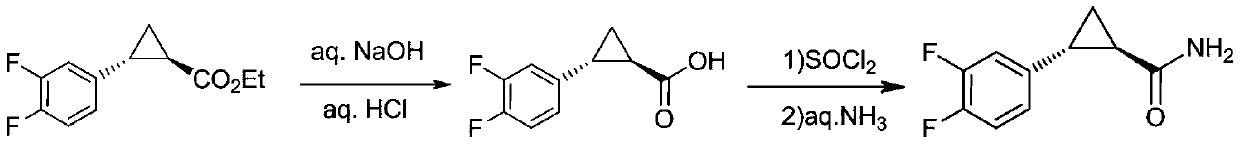
Preparation method of ticagrelor key intermediate aromatic cyclopropanamide
ActiveCN110437092AFew reaction stepsShort reaction cycleOrganic compound preparationOrganic chemistry methodsFormamideHigh pressure
The invention discloses a preparation method of a ticagrelor key intermediate, aromatic cyclopropanamide. The method comprises the following steps: with (1R,2R)-2-(3,4-difluorophenyl)-1-cyclopropyl formate (I) as an initial raw material, carrying out transesterification with formamide under the action of alkali, and meanwhile, maintaining negative pressure or normal pressure to generate a target product, namely, (1R,2R)-2-(3,4-difluorophenyl)-1-cyclopropyl formamide (II). According to the invention, the three-step synthesis reaction in the prior art is simplified by a one-step reaction, and amide is directly generated by formate reaction; therefore, according to the method, reaction steps are saved, the reaction period is shortened, reaction conditions are milder, high-temperature and high-pressure reaction conditions are avoided, and potential safety hazards are greatly reduced. Meanwhile, the raw materials and reagents of the method are low in price, easy to purchase and high in reaction yield, so that the method is low in reaction cost, and industrial production is easy to achieve.
Owner:KAIYUAN HENGTAI PHARMA
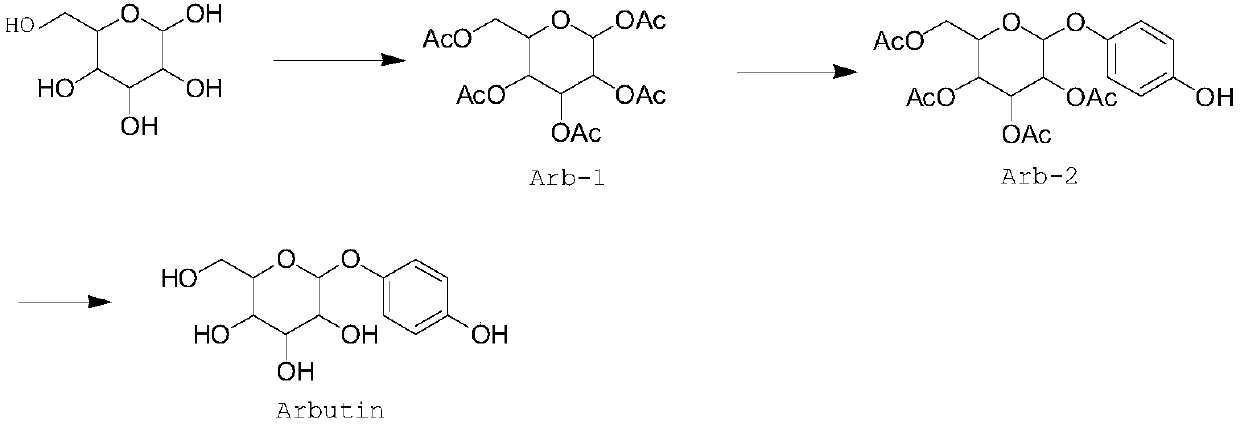
Method of synthesizing arbutin by using solid superacid as catalyst
ActiveCN110343138AReduced activitySave raw materialsPhysical/chemical process catalystsSugar derivativesSodium methoxideArbutin
A dehydrant for a dehydration reaction of a key intermediate for synthesizing arbutin in the existing technical route is boron trifluoride diethyl etherate; ether belongs to a flammable and explosivearticle; another dehydrant is strong acid such as sulfuric acid and p-toluenesulfonic acid; such dehydrant is poor in reaction selectivity and very low in yield, generates much waste acid at the sametime and causes very high environmental protection pressure. For that reason, the invention provides a method of synthesizing arbutin by taking solid superacid as a catalyst. The method comprises thefollowing steps: preparing a solid superacid catalyst, preparing an intermediate Arb-1 by using the solid superacid as the catalyst, preparing an intermediate Arb-2 from Arb-1 by using the solid superacid as the catalyst, recovering and activating the catalyst, and performing reaction and purification using the intermediate Arb-2, methanol and sodium methoxide to form arbutin. The method has the advantages that the method causes little waste gas, waste water and industrial residue, and is green, environmentally friendly, simple and convenient in technical operation and suitable for industrialization.
Owner:和德化学(苏州)有限公司
Preparation method of lysergol
ActiveCN106496220AThe synthetic route is simpleShort synthetic routeOrganic chemistryLysergolSolvent
The invention provides a preparation method of lysergol, and more specifically provides a method for preparing the lysergol directly through a one-step reduction reaction of lysergic acid. A solvent and a reagent adopted in the invention have the advantages of low toxicity, low cost, simple post-processing and the like, and can be recycled and reused; the preparation method is free of special requirement for equipment, and is short in operation working time and low in energy consumption.
Owner:CHONGQING QIANTAI BIOLOGICAL MEDICINE
Synthetic method of bis(2,2,2-trinitro ethyl)-3-6-diamino tetrazine
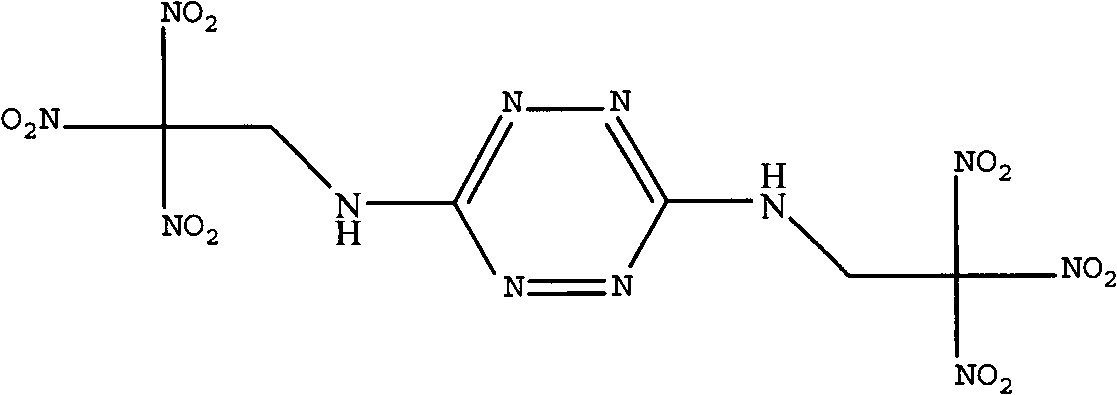
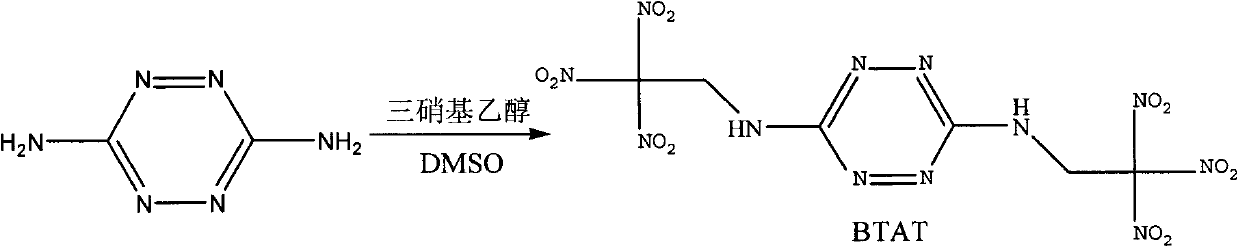
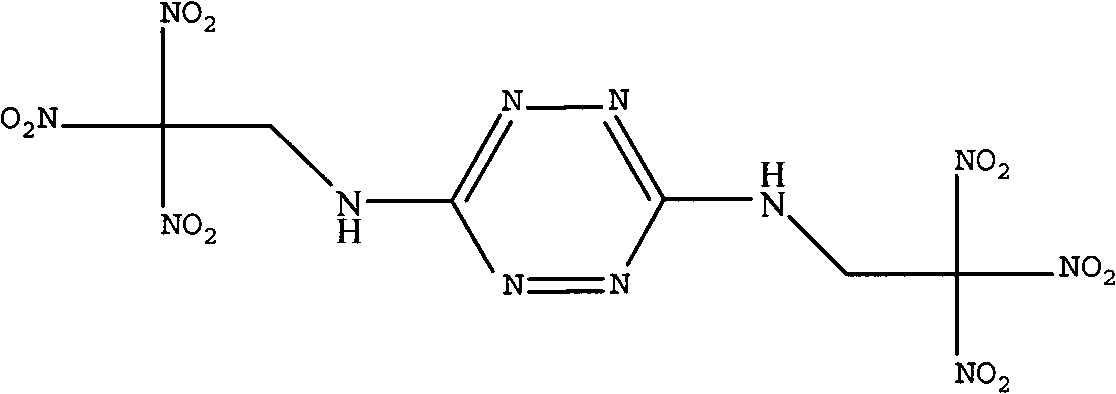
InactiveCN102659704AHigh reaction yieldSimplified processing stepsOrganic chemistryRoom temperatureAqueous solution
The invention discloses a synthetic method of bis(2,2,2-trinitro ethyl)-3-6-diamino tetrazine of the following formula. The method is characterized by using 3,6-diamino tetrazine and trinitro ethanol as raw materials, adding dimethyl sulfoxide of 3,6-diamino tetrazine in an aqueous solution of trinitro ethanol at low temperature under acidic conditions, and reacting at room temperature to obtain bis(2,2,2-trinitro ethyl)-3-6-diamino tetrazine. The invention is mainly used for synthesizing bis(2,2,2-trinitro ethyl)-3-6-diamino tetrazine.
Owner:XIAN MODERN CHEM RES INST
Method for continuously maintaining performance of catalyst for cyclohexene production
PendingCN111804311AReduced activityLower reaction yieldHydrocarbon by hydrogenationCatalyst regeneration/reactivationSulfate zincPtru catalyst
The invention discloses a method for continuously maintaining the performance of a catalyst for cyclohexene production. The method comprises steps: firstly, the feeding amount of a reactor is reduced,a certain amount of a low-activity catalyst is discharged, meanwhile, a corresponding amount of zinc sulfate is added to maintain the concentration of a slurry in the hydrogenation reactor, then a proper amount of a new catalyst is added in batches, the overall activity of the catalyst in the reactor is adjusted, wherein the low-activity catalyst is an old catalyst which operates in the reactor for a long time which is close to the service life. According to the method, two years are taken as a replacement period, the catalyst in the reactor is quantitatively replaced, and the total replacement amount is 30-60Wt% of the total amount of the catalyst in the reactor. According to the method disclosed by the invention, the unit consumption of the new catalyst is reduced, the catalyst adjusting time is shortened, the cost is effectively reduced, and the continuity of a production system is maintained.
Owner:HENAN SHENMA NYLON CHEM
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com