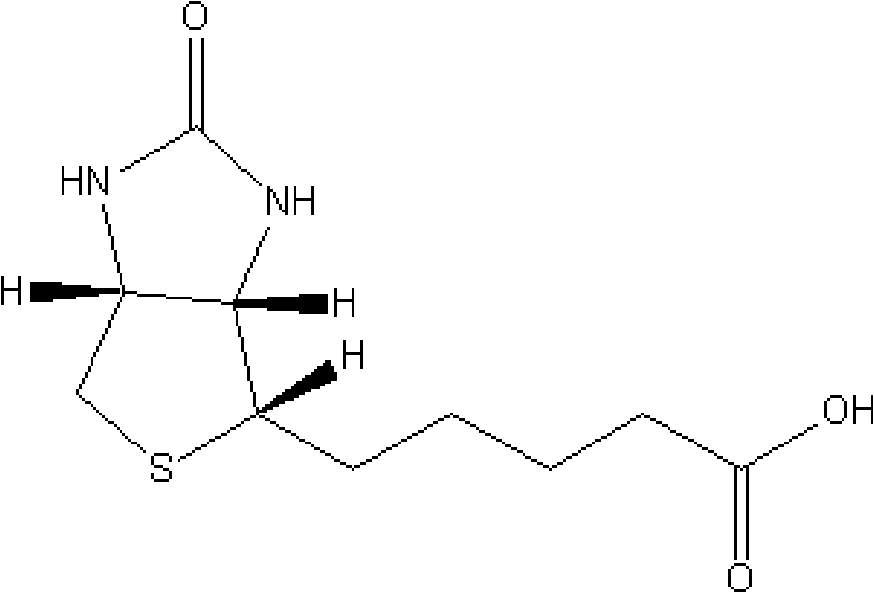
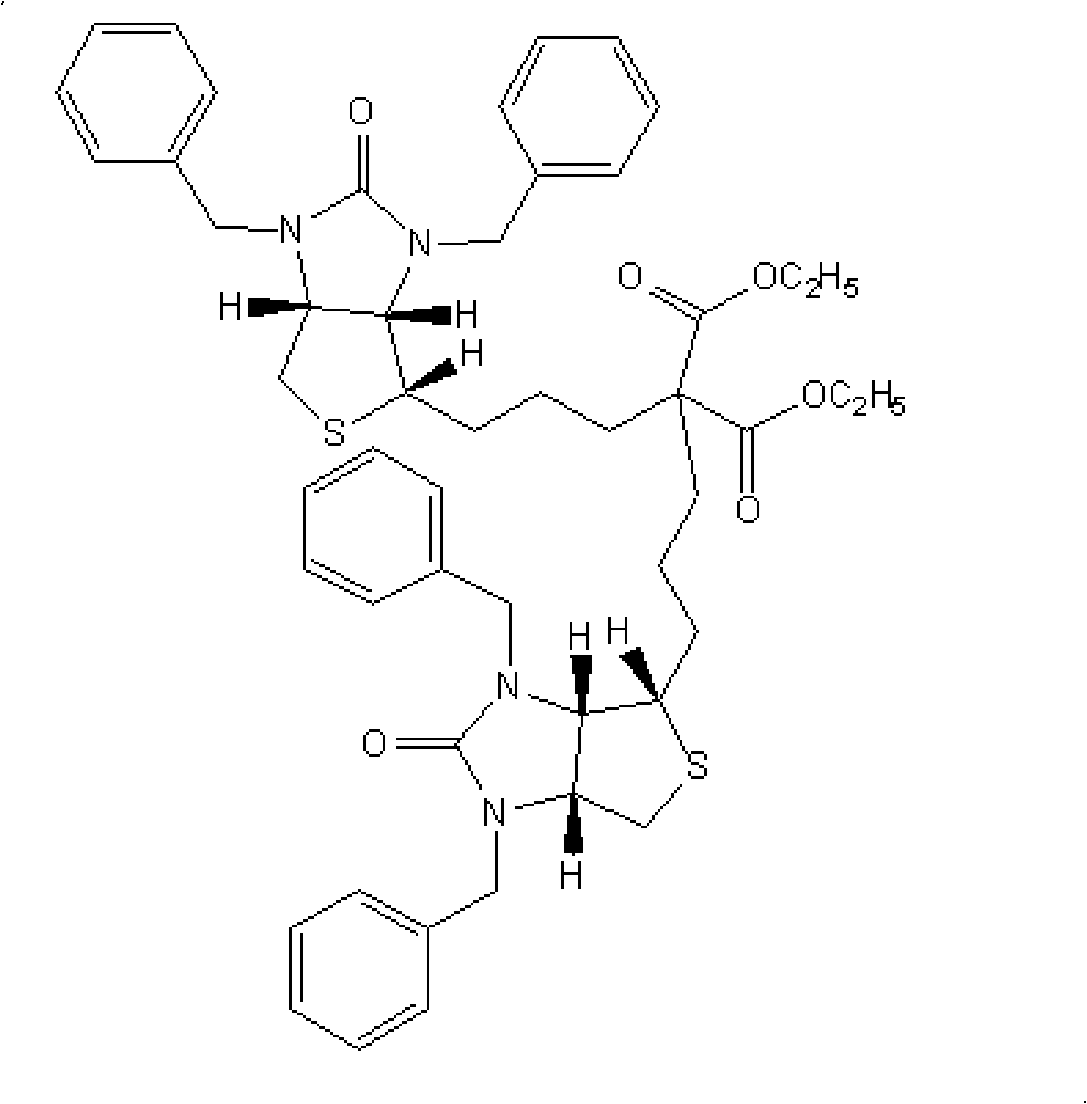
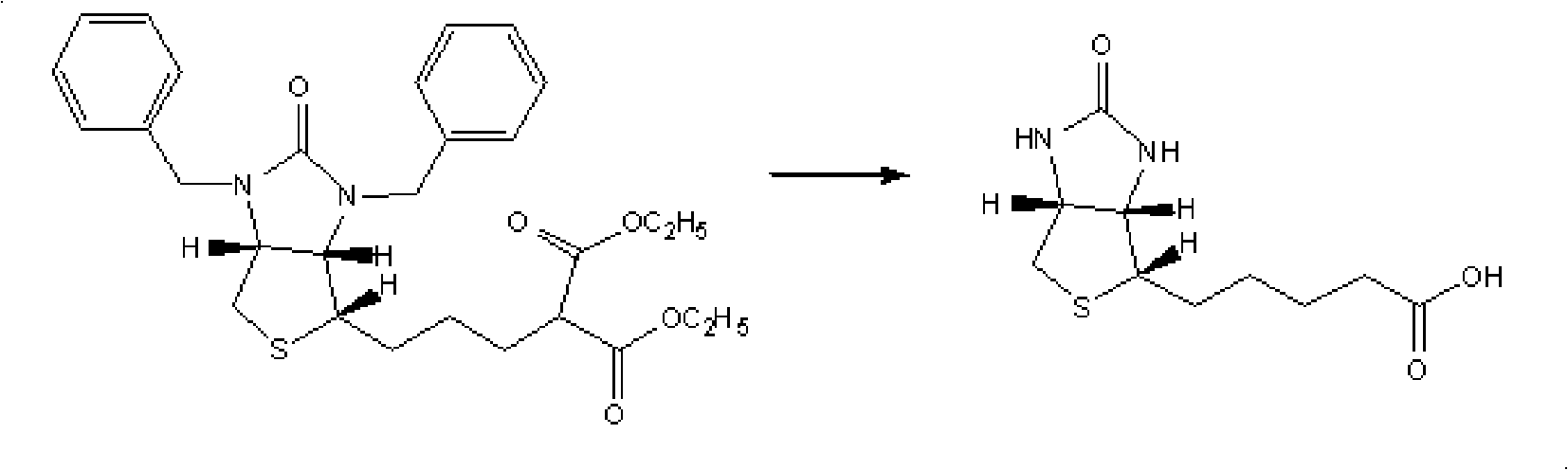
Preparation method of d-biotin
A biotin, bibenzyl biotin technology, applied in the direction of organic chemistry, etc., can solve the problems such as the decrease of d-biotin yield, the influence of d-biotin yield, the decrease of valeric acid yield, etc. High product yield and the effect of reducing dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a 250ml three-necked flask equipped with mechanical stirring, a thermometer, and a reflux condenser, 13.8g (0.1mol) of ethyl 2,2-dicyanoacetate, 13.8g (0.1mol) of potassium carbonate, and N,N-dimethyl Acetyl formamide 100ml, heated to 60°C for 0.5 hours under stirring, and added (3aR, 8aS, 8bS)-1,3-dibenzyl-2-oxo-decahydroimidazo[4,5-c at 60°C ]Thieno[1,2-a]sulfonium bromide 44.5g (0.1mol), stirred and reacted at 60°C for 1 hour, raised the temperature to 100-105°C for 1 hour, after the reaction was completed, N,N -Dimethylformamide, to obtain a viscous substance, add 50ml of water, add 300ml of toluene, stir to dissolve, adjust pH=5.0 with hydrochloric acid, separate layers, wash the toluene layer twice with 10ml×2 water, and extract the combined water layer with 100ml×2 toluene Twice, the toluene layer was combined, fully dehydrated, the toluene was evaporated under reduced pressure, and 50ml of petroleum ether (90-120°C) was added to the residue, stirred for 1 hou...
Embodiment 2
[0040] In a 250ml three-necked flask equipped with mechanical stirring, a thermometer, and a reflux condenser, 13.8g (0.1mol) of ethyl 2,2-dicyanoacetate, 13.8g (0.1mol) of potassium carbonate, and N,N-dimethyl Acetyl formamide 100ml, heated to 60°C for 0.5 hours under stirring, and added (3aR, 8aS, 8bS)-1,3-dibenzyl-2-oxo-decahydroimidazo[4,5-c at 60°C ]Thieno[1,2-a]sulfonium chloride 40.1g (0.1mol), stirred and reacted at 60°C for 1 hour, raised the temperature to 100-105°C for 1 hour, after the reaction was completed, evaporated under reduced pressure to remove N,N -Dimethylformamide, to obtain a viscous substance, add 50ml of water, add 300ml of toluene, stir to dissolve, adjust pH=5.0 with hydrochloric acid, separate layers, wash the toluene layer twice with 10ml×2 water, and extract the combined water layer with 100ml×2 toluene Twice, the toluene layer was combined, fully dehydrated, the toluene was evaporated under reduced pressure, and 50ml of petroleum ether (90-120°C...
Embodiment 3
[0042] In a 250ml three-neck flask equipped with mechanical stirring, a thermometer, and a reflux condenser, put 13.8g (0.1mol) of ethyl 2,2-dicyanoacetate, 20.2g (0.2mol) of triethylamine, and N-methylpyrrolidone 100ml, heated to 60°C for 0.5 hours under stirring, then added (3aR, 8aS, 8bS)-1,3-dibenzyl-2-oxo-decahydroimidazo[4,5-c]thieno at 60°C [1,2-a] 44.5g (0.1mol) of sulfonium bromide, stirred and reacted at 60°C for 1 hour, raised the temperature to 100-105°C and reacted for 1 hour, after the reaction was completed, N-methylpyrrolidone, Triethylamine, to obtain a viscous substance, add 50ml of water, add 300ml of toluene, stir to dissolve, adjust pH=5.0 with hydrochloric acid, separate layers, wash the toluene layer twice with 10ml×2 water, and extract the combined water layer twice with 100ml×2 toluene, Combine the toluene layers, fully dehydrate, evaporate the toluene under reduced pressure, add 50ml of petroleum ether (90-120°C) to the residue, stir for 1 hour, cool ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com