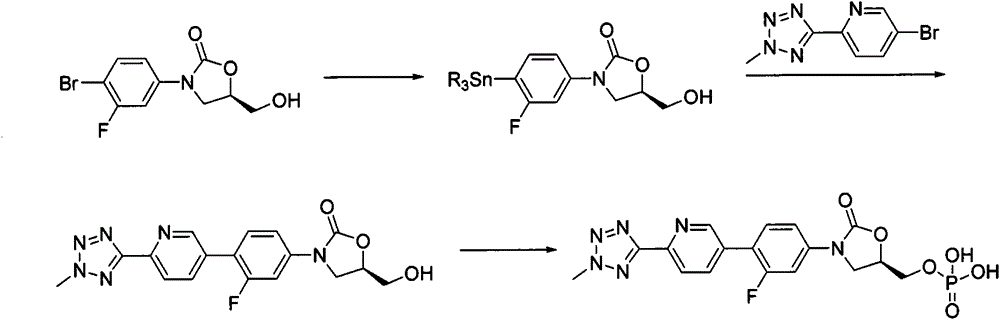
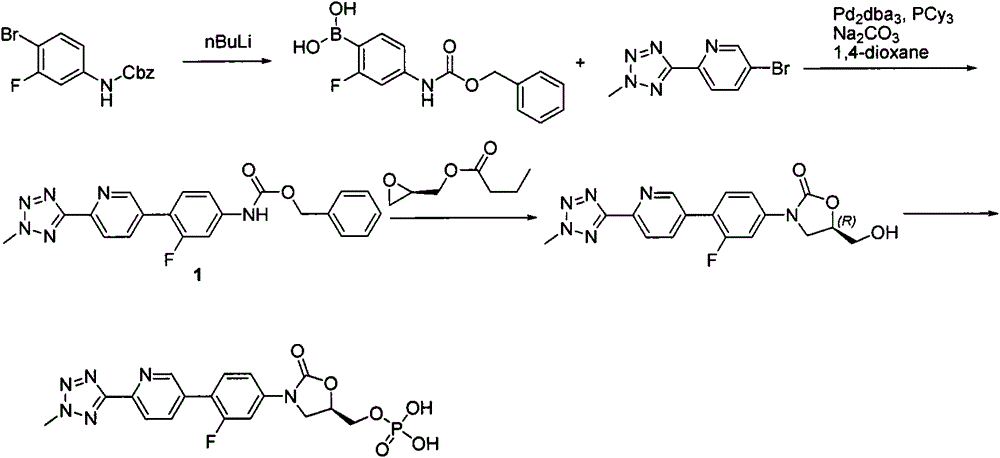
New synthesis process of oxazolinone antibiotic
A new synthesis and compound technology, applied in the new synthesis field of tedizolid intermediates, can solve the problems of industrialized production, hidden dangers of large-scale production safety, insufficient process robustness, etc., to ensure quality and continuous supply, increase Process robustness, cost reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
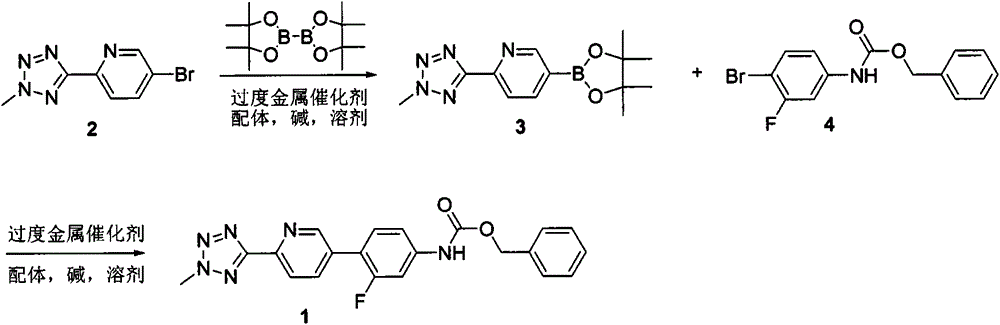
[0031] Example 1: Synthesis of benzyl 3-fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenylcarbamate (Compound 1)
[0032]
[0033] Put compound 2 (2.4g, 10mmol), potassium acetate (2.9g, 30mmol) and pinacol diboron (3g, 12mmol) in a 500mL three-necked flask, add 1,4-dioxane (70mL) and Pd (dppf) 2 Cl 2 ·CH 2 Cl 2 (800mg, 1mmol). After nitrogen protection, heat to 80°C and react for 3 hours. After LC / MS detection confirmed that compound 2 was completely converted into compound 3, compound 4 (2.93g, 9mmol), Pd(dppf) were added 2 Cl 2 ·CH 2 Cl 2 (800mg, 1mmol), potassium carbonate (3.45g, 25mmol) and water (20mL), protected by nitrogen again, heated to 80°C for 12 hours. LCMS confirmed that the reaction was complete. Suction filtration was performed to remove the solid material. The obtained filtrate was evaporated under reduced pressure on a rotary evaporator to remove 1,4-dioxane, and then extracted with dichloromethane (100 mL×2), and the organic phases were combined and...
Embodiment 2
[0034] Example 2: Synthesis of benzyl 3-fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenylcarbamate (Compound 1)
[0035]
[0036] Put compound 2 (120g, 0.5mol), potassium acetate (145g, 0.5mol) and pinacol diboron (150g, 0.6mol) in a 10L four-neck flask, and add 1,4-dioxane (3L) And Pd(dppf) 2 Cl 2 ·CH 2 Cl 2 (20g, 25mmol). After nitrogen protection, heat to 80°C and react for 3 hours. After LC / MS detection confirmed that compound 2 was completely converted into compound 3, compound 4 (146g, 0.45mol), potassium carbonate (173g, 1.2mol) and water (1L) were added, re-protected with nitrogen, and heated to 80℃ for 12 hours. . LCMS confirmed that the reaction was complete. Suction filtration was performed to remove solid materials. The obtained filtrate was evaporated under reduced pressure on a rotary evaporator to remove 1,4-dioxane, 500 mL of ethanol was added to the water phase, and the slurry was beaten overnight. After suction filtration, the filter cake was washed...
Embodiment 3
[0037] Example 3: Synthesis of benzyl 3-fluoro-4-(6-(2-methyl-2H-tetrazol-5-yl)pyridin-3-yl)phenylcarbamate (Compound 1)
[0038] Step 1: 5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-(2-methyl-2H-tetrazole- Synthesis of 5-yl)pyridine
[0039]
[0040] Put compound 2 (2.4g, 10mmol), potassium acetate (2.9g, 30mmol) and pinacol diboron (3g, 12mmol) in a 250mL three-necked flask, add 1,4-dioxane (70mL) and Pd (dppf) 2 Cl 2 ·CH 2 Cl 2 (800mg, 1mmol). After nitrogen protection, heat to 80°C and react for 3 hours. After LC / MS detection confirmed that compound 2 was completely converted into compound 3, the temperature of the reaction system was lowered to room temperature, filtered, and the filtrate was evaporated to dryness. Add 50 mL of water and extract with ethyl acetate. The organic phases were combined, washed with saturated brine (200 mL×1), and dried with anhydrous sodium sulfate. Suction filtration, and the filtrate was evaporated to dryness under reduced pressure with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com