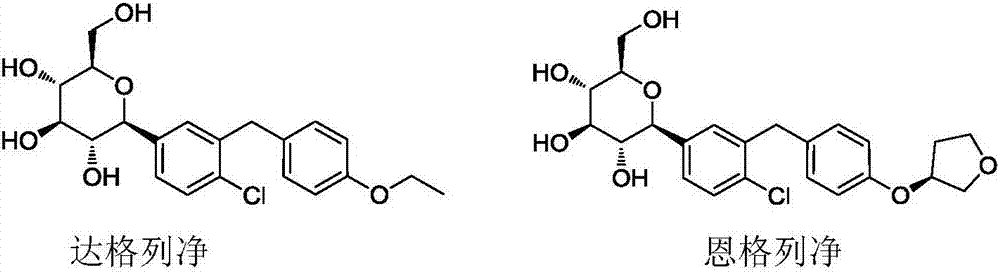
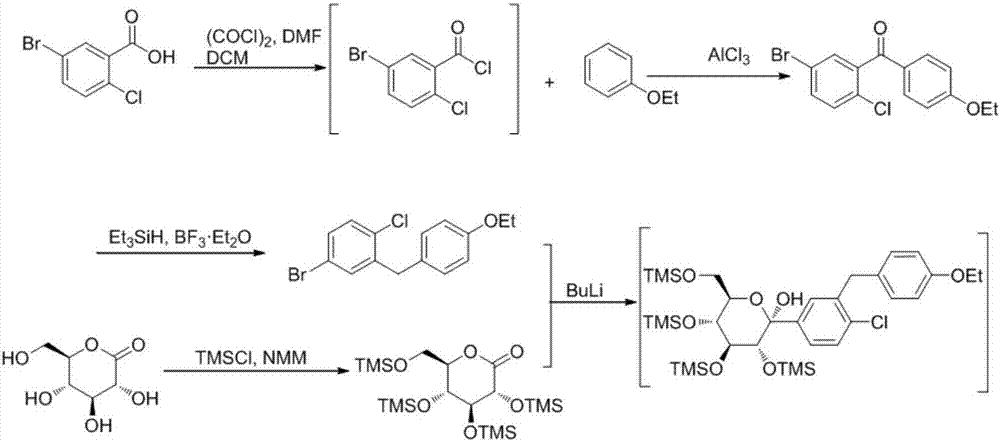
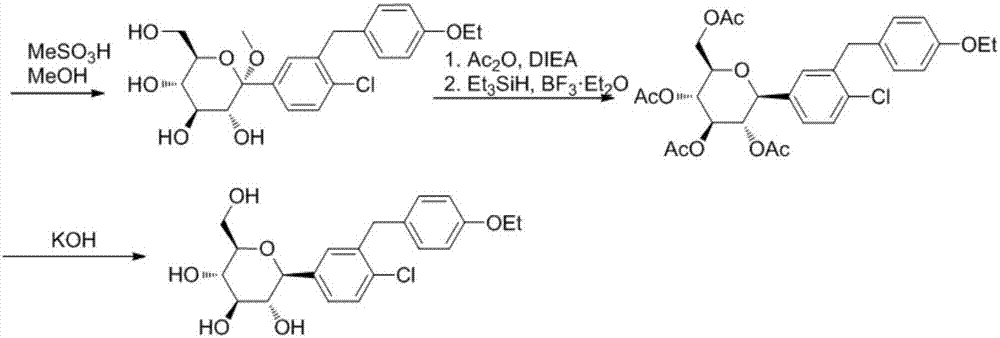
Preparation methods of SGLT-2 diabetes inhibitors and intermediates thereof
A technology of SGLT-2, 1. SGLT-2 is applied in the field of preparation of SGLT-2 inhibitors, which can solve the problems of high route cost, extremely high reaction temperature, high moisture requirements, and difficult operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (5-Bromo-2-chlorophenyl)(4-ethoxyphenyl)methanol
[0058]
[0059] Add (5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanone 1a (33.96g, 100mmol), tetrahydrofuran (170mL), methanol (34mL) into the three-necked flask, stir well and cool to 0~ At 5°C, sodium borohydride (4.54 g, 120 mmol) was added in batches, and after the addition was completed, the temperature was slowly raised to room temperature for 1-2 hours. After the reaction is completed, cool to 0-5°C and add 0.5mol / L dilute hydrochloric acid (170mL) to quench the reaction, add ethyl acetate (170mL) to the aqueous phase to extract twice, combine the organic phases, wash once with saturated brine (170mL), and dry over sodium sulfate , after concentrating, beat with ethyl acetate petroleum ether, filter, wash with a small amount of petroleum ether, and dry to obtain compound (5-bromo-2-chlorophenyl)(4-ethoxyphenyl)methanol 2a (31.43g, 92%) .
[0060] Here the reducing agent sodium borohydride can be replaced by b...
Embodiment 2
[0062] (5-iodo-2-chlorophenyl)(4-ethoxyphenyl)methanol
[0063]
[0064] Add (5-iodo-2-chlorophenyl)(4-ethoxyphenyl)methanone 1b (38.66g, 100mmol), tetrahydrofuran (193mL), methanol (39mL) into the three-necked flask, stir well and cool to 0~ At 5°C, potassium borohydride (6.47 g, 120 mmol) was added in batches, and after the addition was completed, the temperature was slowly raised to room temperature for 2-3 hours. After the reaction is completed, cool to 0-5°C and add 0.5mol / L dilute hydrochloric acid (193mL) to quench the reaction, add ethyl acetate (193mL) to the aqueous phase to extract twice, combine the organic phases, wash once with saturated brine (193mL), and dry over sodium sulfate , concentrated with ethyl acetate petroleum ether, filtered, washed with a small amount of petroleum ether, and dried to obtain compound (5-iodo-2-chlorophenyl)(4-ethoxyphenyl)methanol 2b (34.98g, 90%) .
Embodiment 3
[0066] 4-Bromo-1-chloro-2-((4-ethoxyphenyl)(methoxy)methyl)benzene
[0067]
[0068] Add 2a (34.16 g, 100 mmol), toluene (170 mL) and 15% methanolic hydrochloric acid solution (102 mL) into a three-necked flask, stir well and heat to 65-70° C. to react overnight. After the reaction was completed, cool to room temperature, add water (170mL) to separate the liquids, extract the water phase with toluene (170mL) once more, combine the organic phases and wash with saturated brine once (170mL), dry over sodium sulfate, filter, concentrate and wash with ethyl acetate The compound 4-bromo-1-chloro-2-((4-ethoxyphenyl)(methoxy)methyl)benzene 4a (32.72 g, 92%) was obtained by recrystallization from petroleum ether mixed solvent.
[0069] MS(ESI)m / z=355.1[M+H] + , 1 HNMR (CDCl3, 400MHz) δ7.80(d, J=2.4Hz, 1H), 7.35-7.25(m, 3H), 7.19(d, J=8.5Hz, 1H), 6.90-6.85(m, 2H), 5.66(s,1H),4.03(q,J=6.9Hz,2H),3.40(s,3H),1.29(t,J=6.8Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com