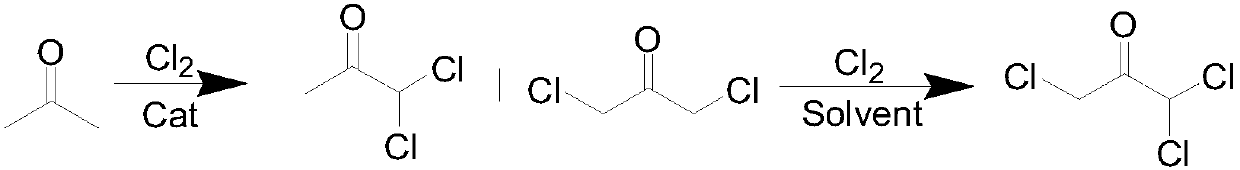
Method for preparing 1,1,3-trichloroacetone through high-selectivity chlorination of acetone
A high-selectivity, trichloroacetone technology, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of poor synthesis technology, low preparation efficiency, and complicated operation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0028] Example 1:
[0029] A method for preparing 1,1,3-trichloroacetone by highly selective chlorination of acetone, the preparation method comprising the following steps:
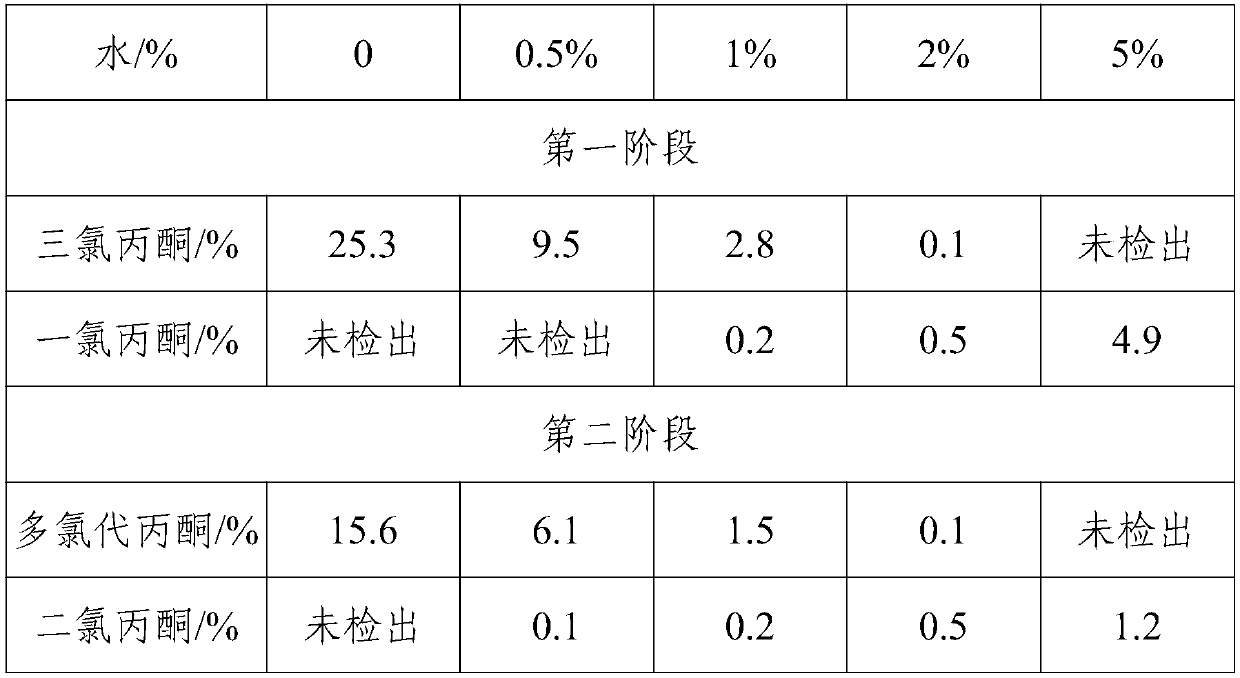
[0030] (1) Add 90 g of acetone to the container, add 1 g of triethylamine under stirring, then add 0.5 g of water, and control the temperature at 30° C. to feed chlorine gas to carry out the first chlorination stage;
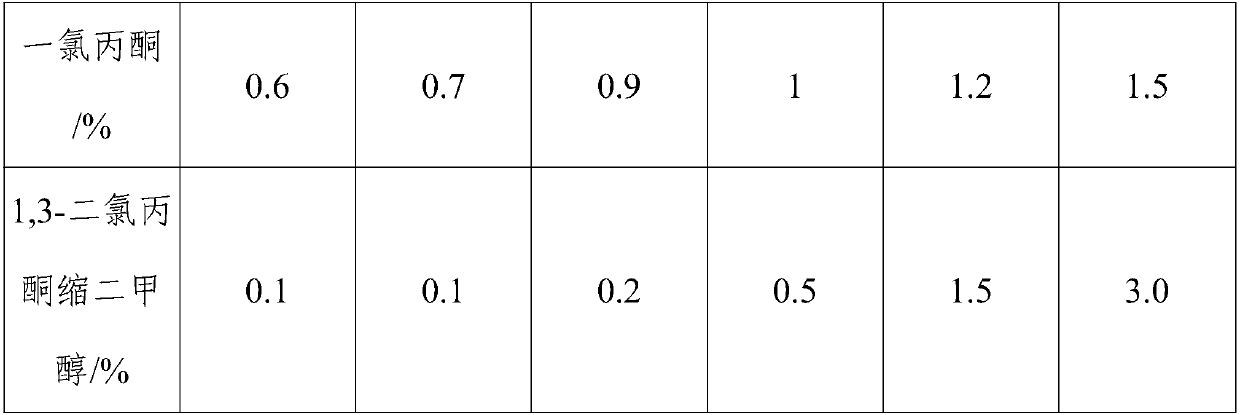
[0031] (2) When the content of monochloroacetone in the reaction solution is lower than 1%, add alcohol compounds, and control the temperature to be 30° C. to carry out the second chlorination stage;
[0032] (3) When the dichloroacetone in the reaction solution is completely consumed, the reaction is stopped, air is blown in to blow out residual chlorine gas, and the solvent is removed by distillation under reduced pressure to obtain 1,1,3-trichloroacetone.
Example Embodiment
[0033] Example 2:
[0034] A method for preparing 1,1,3-trichloroacetone by highly selective chlorination of acetone, the preparation method comprising the following steps:
[0035] (1) Add 105g of acetone in the container, add 3g of triethylamine under stirring conditions, then add 3g of water, control the temperature at 60°C and feed chlorine gas to carry out the first chlorination stage;
[0036] (2) When the content of monochloroacetone in the reaction solution is lower than 1%, add an alcohol compound, and control the temperature to be 50° C. to carry out the second chlorination stage;
[0037] (3) When the dichloroacetone in the reaction solution is completely consumed, the reaction is stopped, air is blown in to blow out residual chlorine gas, and the solvent is removed by distillation under reduced pressure to obtain 1,1,3-trichloroacetone.
Example Embodiment
[0038] Example 3:
[0039] A method for preparing 1,1,3-trichloroacetone by highly selective chlorination of acetone, the preparation method comprising the following steps:
[0040] (1) Add 100 g of acetone to the container, add 1 g of triethylamine under stirring, then add 2 g of water, and control the temperature at 40° C. to feed chlorine to carry out the first chlorination stage;
[0041] (2) When the content of monochloroacetone in the reaction solution is lower than 1%, add alcohol compounds, and control the temperature to be 35° C. to carry out the second chlorination stage;
[0042] (3) When the dichloroacetone in the reaction solution is completely consumed, the reaction is stopped, air is blown in to blow out residual chlorine gas, and the solvent is removed by distillation under reduced pressure to obtain 1,1,3-trichloroacetone.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com