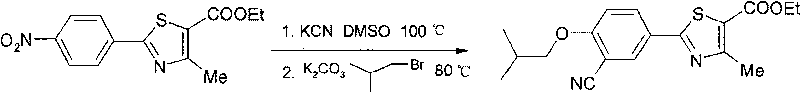Preparation method of important intermediate of novel febuxostat
A technology of ethyl methyl and thiazole carboxylate, which is applied in the chemical and pharmaceutical fields, and can solve problems such as high cost, great environmental hazards, and low reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
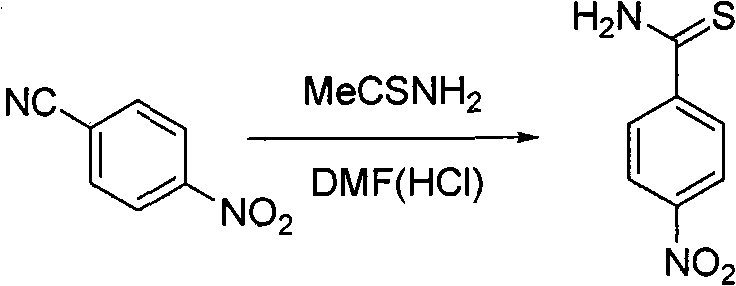
[0035] The preparation method of p-nitrophenylsulfamide:
[0036] Put p-nitrobenzonitrile (10g) and thioacetamide (12.7g) into 85mL DMF (10% HCl) at room temperature, stir for 5mins and then raise the temperature to 100°C, keep warm until TLC traces no raw materials (about 3h), Cool, pour into 30g of ice, filter, wash the filter residue with water (20×3), and dry to obtain 10g of light yellow solid with a yield of 81%.
Embodiment 2
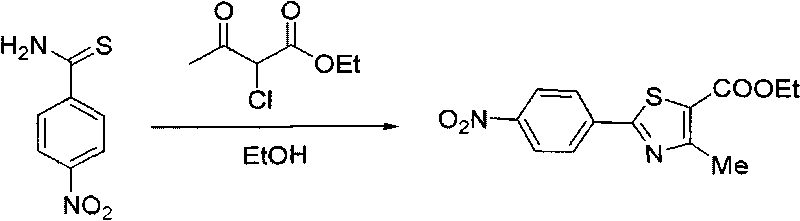
[0038] The preparation method of 2-(4-nitrophenyl)-4-methyl-5-thiazolecarboxylic acid ethyl ester:
[0039] Take p-nitrophenylsulfamide (10g) and add it to 50mL of ethanol, add 2-chloro-ethyl acetoacetate (11.5g), heat up to reflux under stirring, cool down after TLC tracking until there is no raw material, filter to obtain 13g of white solid , yield 80%.
Embodiment 3
[0041] The preparation method of 2-[3-cyano-(2-isobutyloxy)phenyl]-4-methyl-5-thiazolecarboxylic acid ethyl ester:
[0042] Take ethyl 2-(4-nitrophenyl)-4-methyl-5-thiazolecarboxylate (14.8g) and potassium cyanide (4.9g) into 150mL DMSO at room temperature, stir, and heat to 102°C. Insulate the reaction until TLC traces no raw materials (about 2 ~ 3h), cool, add K 2 CO 3 (3.8g), bromoisobutane (16g), be warming up to 80 ℃ under stirring, insulation reaction is followed to TLC reaction completely (about 7
[0043] h), cooled, poured into ice, extracted with ethyl acetate, washed the organic layer with water, dried, and spin-dried to obtain a yellow solid, which was recrystallized from ethyl acetate to obtain 15 g of off-white solid with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com