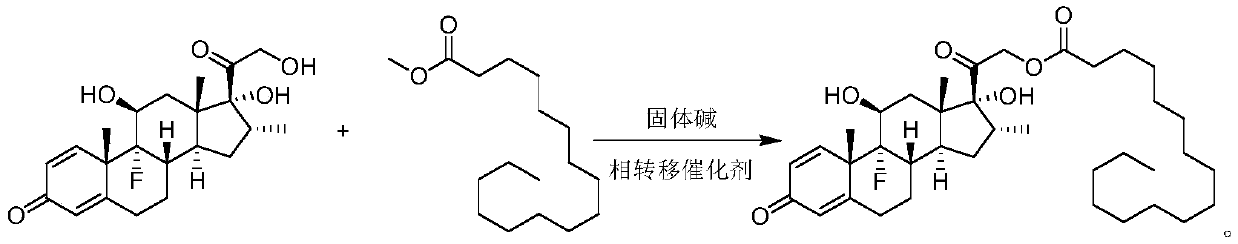
Synthesis method of dexamethasone palmitate
A technology of dexamethasone palmitate and a synthesis method, applied in the directions of steroids, organic chemistry, etc., can solve the problems of complicated separation steps of catalysts and products, unreusable catalysts, discharge pollution of corrosive waste water, etc. The separation, post-treatment and purification of the reaction system are convenient, and the effect of not corroding equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
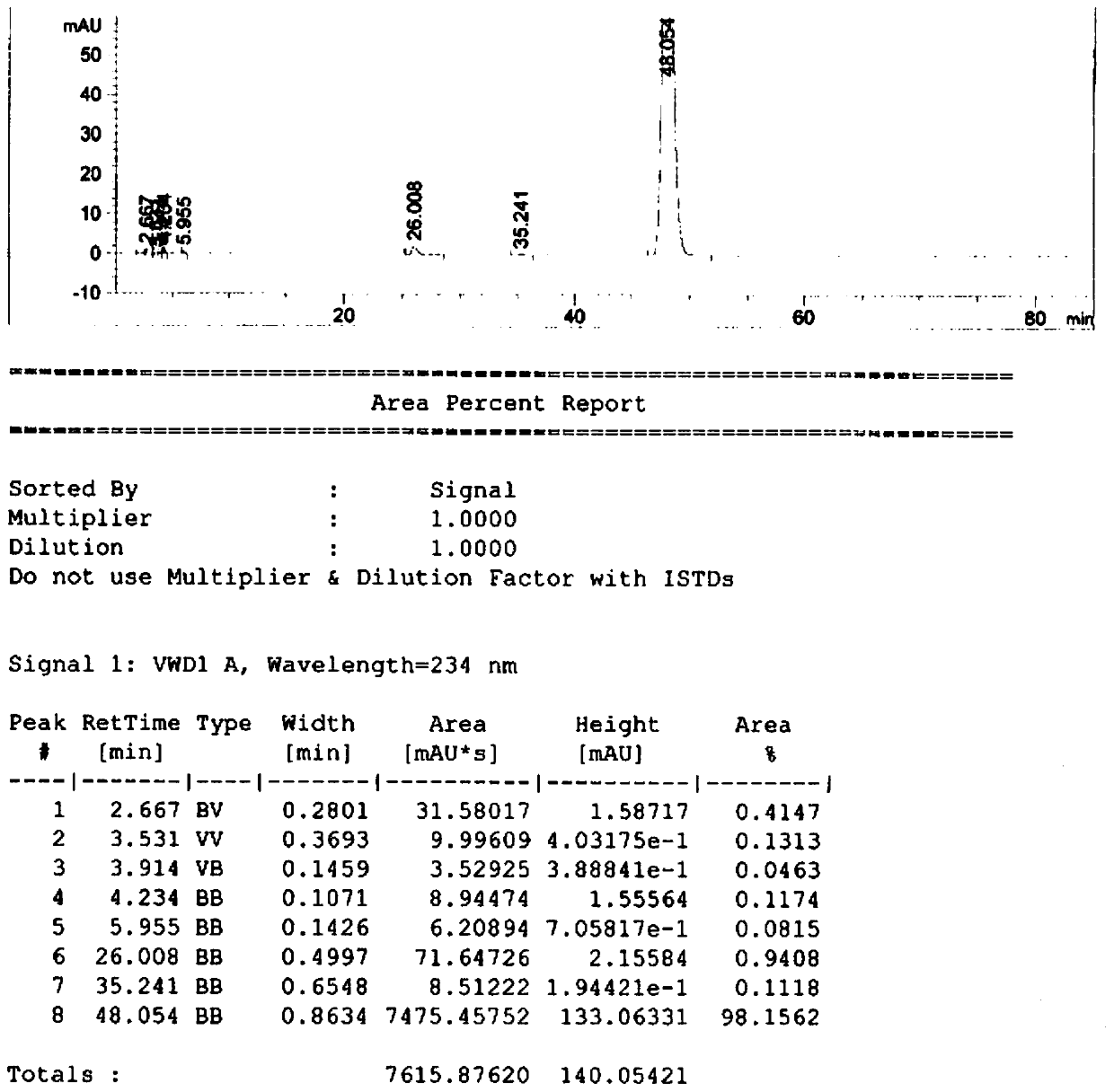
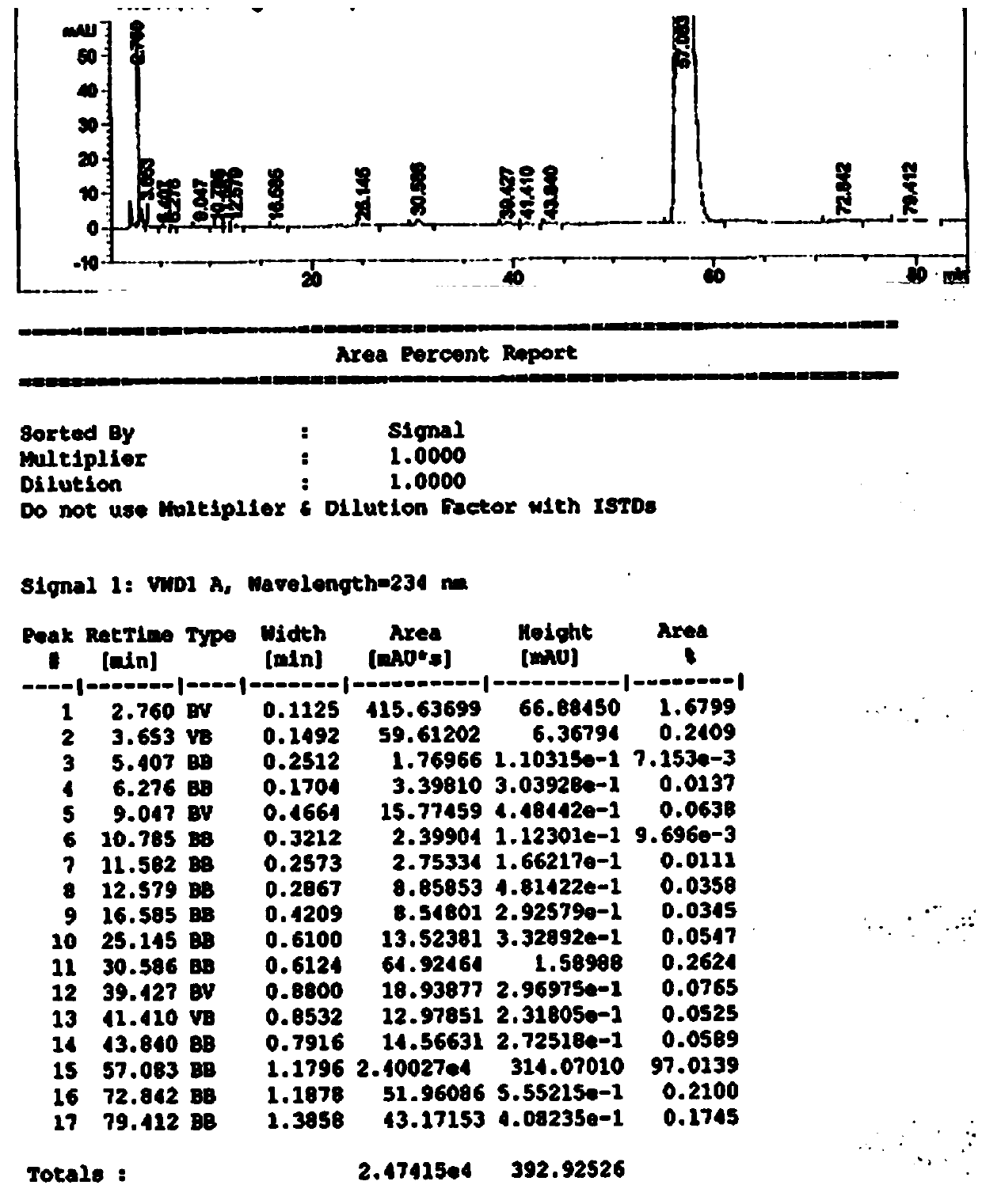
Image
Examples
Embodiment 1
[0036] Under the protection of nitrogen, add 540g (2.0mol) of methyl palmitate to a 1000mL three-necked flask, raise the temperature to about 40°C, add 60g (0.153mol) of dexamethasone, 12g of cesium oxide supported on γ-alumina, and 3g of polyethylene glycol Glycol 400, adjust the vacuum degree to 0.07MPa, heat up to 80°C, react for 3h, TLC monitors the completion of the raw material reaction (developing solvent, petroleum ether: ethyl acetate = 1:1), filter while hot, wash the filter with 90g of acetone Cake, the filtrate was cooled to 0°C, crystallized, and 88.7 g of the product was obtained by filtration, with a yield of 92% and a purity of 99.60%. The filter cake is dried and recycled, and the mother liquor after crystallization and filtration is evaporated to remove acetone to obtain a mixture of methyl palmitate and the product, which is recycled.
Embodiment 2
[0038] Under nitrogen protection, add 540g (2.0mol) of methyl palmitate into a 1000mL three-necked flask, raise the temperature to about 40°C, add 60g (0.153mol) of dexamethasone, 3g of calcium oxide loaded on molecular sieves, 0.6g of polyethylene glycol Alcohol 600, adjust the vacuum to 0.05MPa, heat up to 50°C, react for 10h, TLC monitors the completion of the reaction of raw materials (developing solvent, petroleum ether: ethyl acetate = 1:1), filter while hot, and wash the filter cake with 225g methanol , the filtrate was cooled to -10°C, crystallized, and 87g of the product was obtained by filtration, with a yield of 90.25% and a purity of 99.30%. The filter cake is dried and reused mechanically, the mother liquor after crystallization and filtration, and the mixture of methyl palmitate and the product obtained after distilling off methanol is recycled mechanically.
Embodiment 3
[0040] Under nitrogen protection, add 540g (2.0mol) of methyl palmitate into a 1000mL three-necked flask, raise the temperature to about 40°C, add 60g (0.153mol) of dexamethasone, 6g of potassium oxide loaded on molecular sieves, 1.8g of polyethylene glycol Alcohol 800, adjust the vacuum degree to 0.1MPa, raise the temperature to 70°C, react for 6h, monitor the completion of the raw material reaction by TLC (developing solvent, petroleum ether: ethyl acetate = 1:1), filter while hot, and wash the filter cake with 300g ethanol , the filtrate was cooled to 10°C, crystallized, and 85.8 g of the product was obtained by filtration, with a yield of 89% and a purity of 99.80%. The filter cake is dried and reused mechanically. The mother liquor after crystallization and filtration is evaporated to remove ethanol and the mixture of methyl palmitate and the product is recycled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com