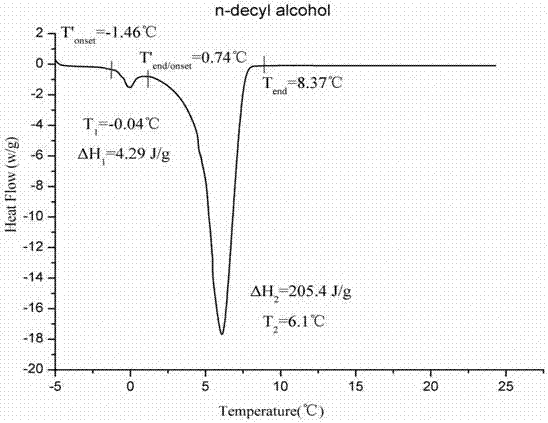
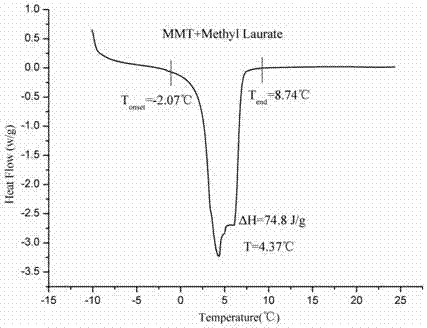
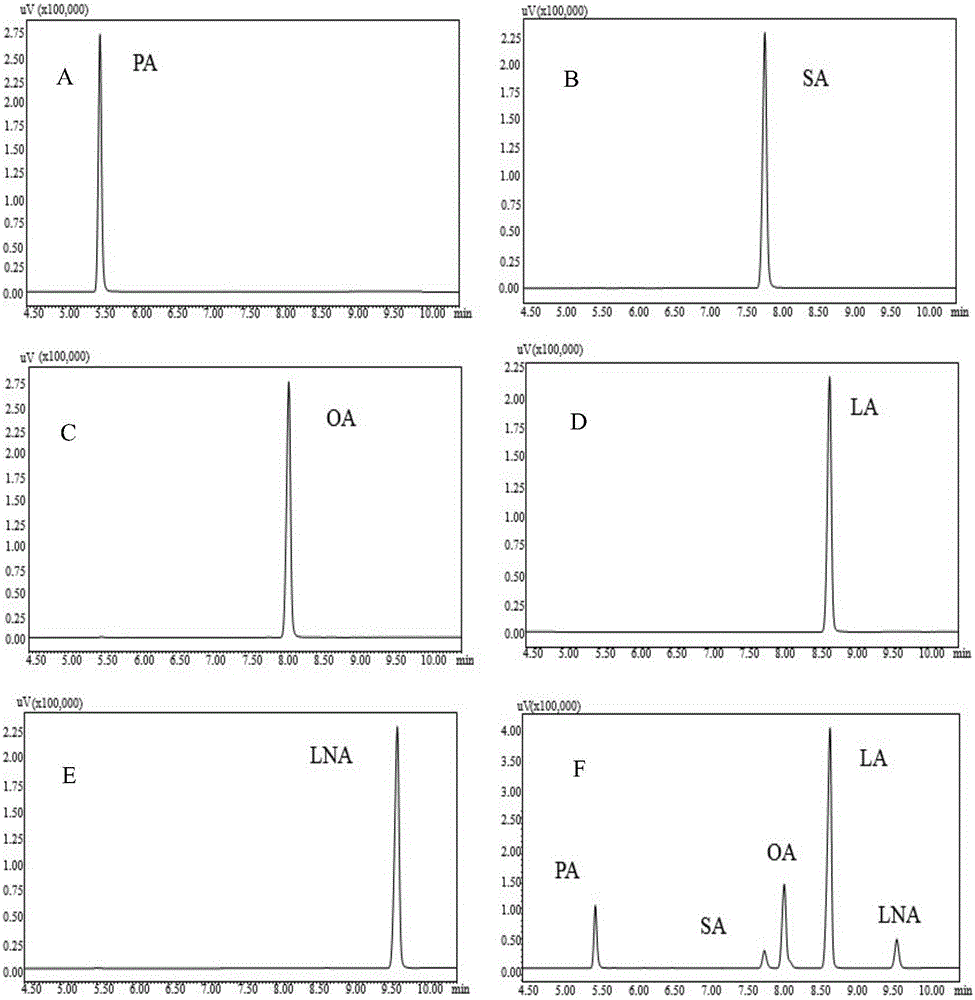
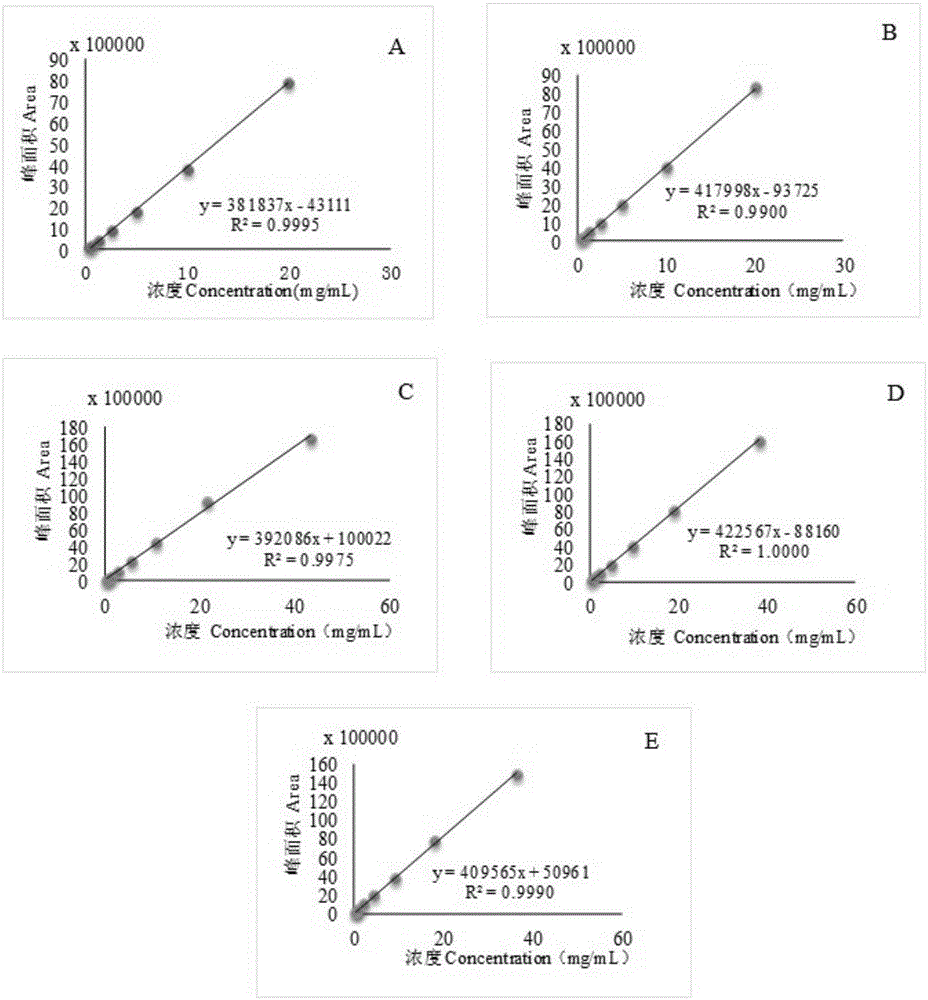
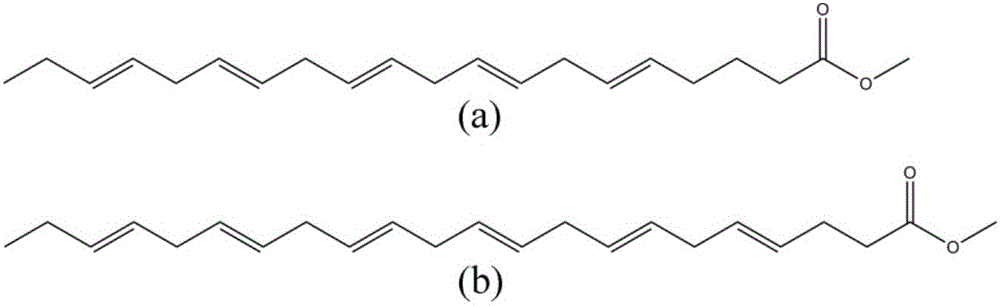
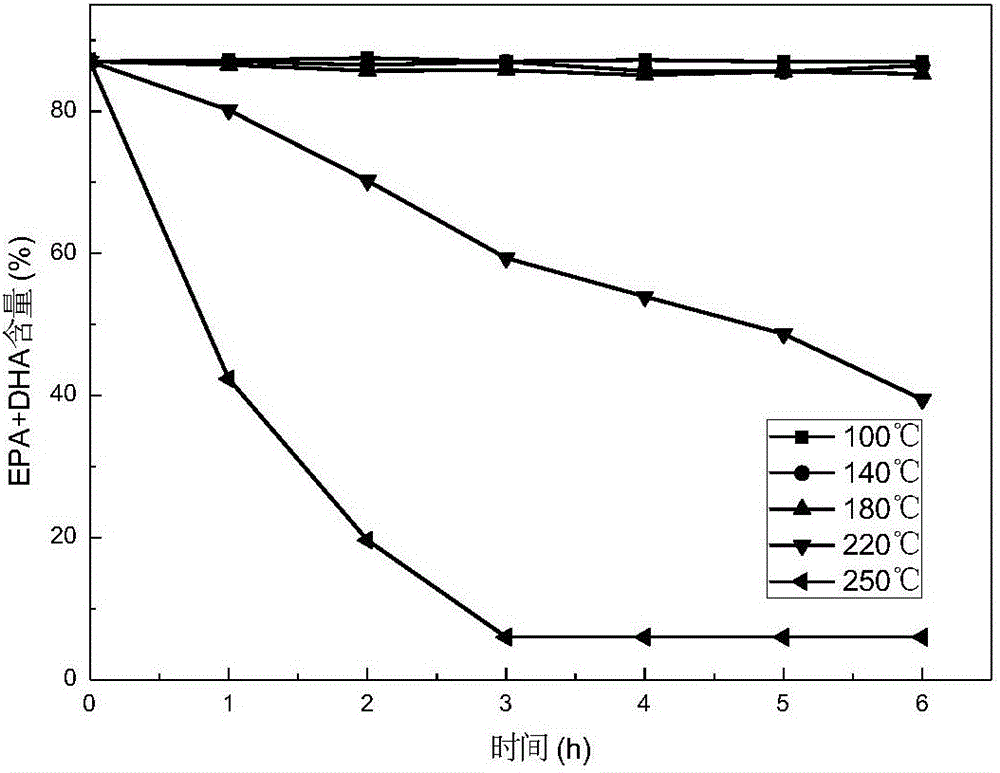
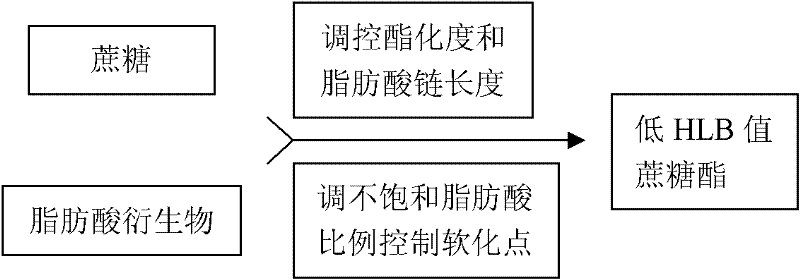
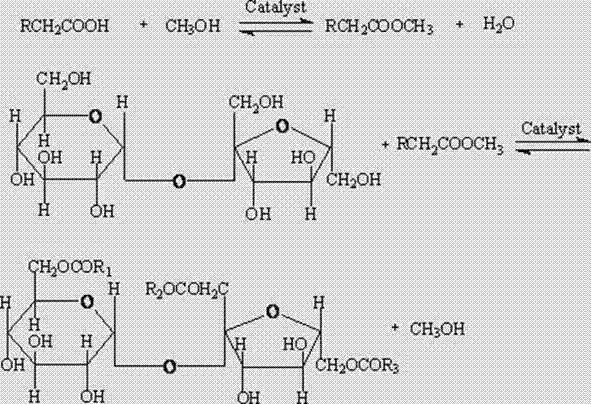
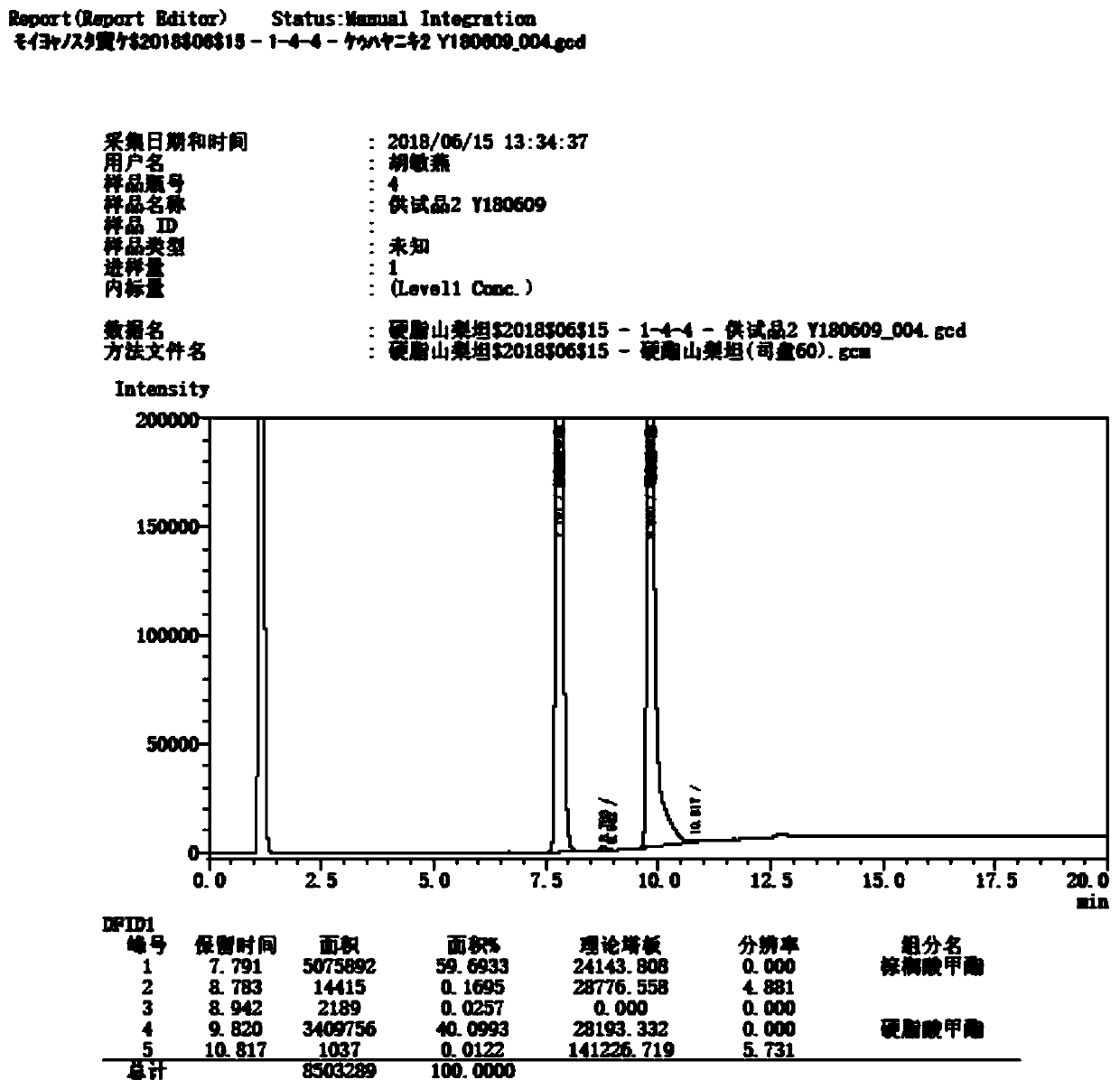
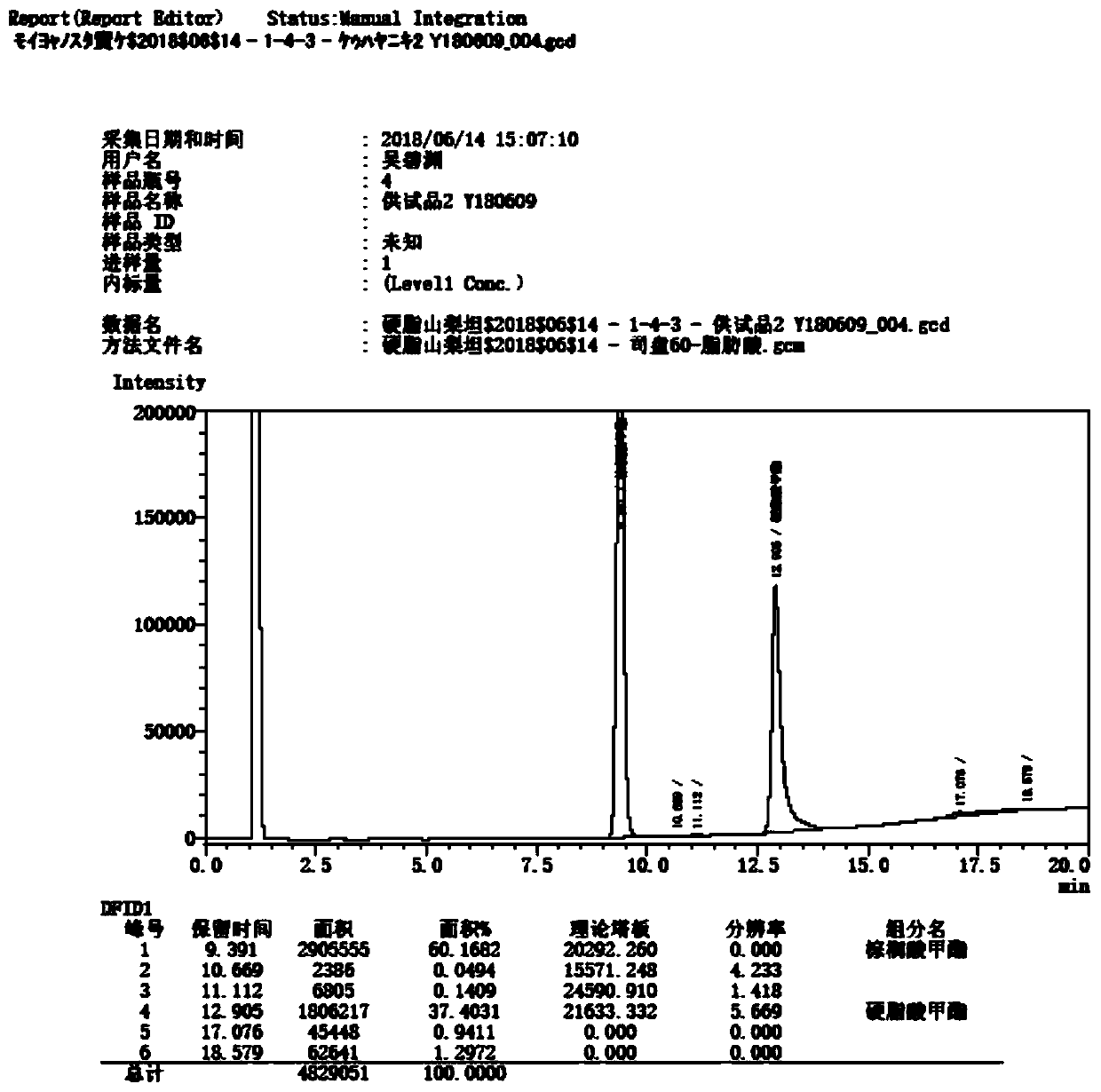
Patents
Literature
115 results about "Methyl palmitate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methyl palmitate. CH3(CH2)14COOCH3 A colorless liquid with a boiling point of 211.5°C; soluble in alcohol and ether; used in the manufacture of detergents, resins, plasticizers, lubricants, and animal feed.
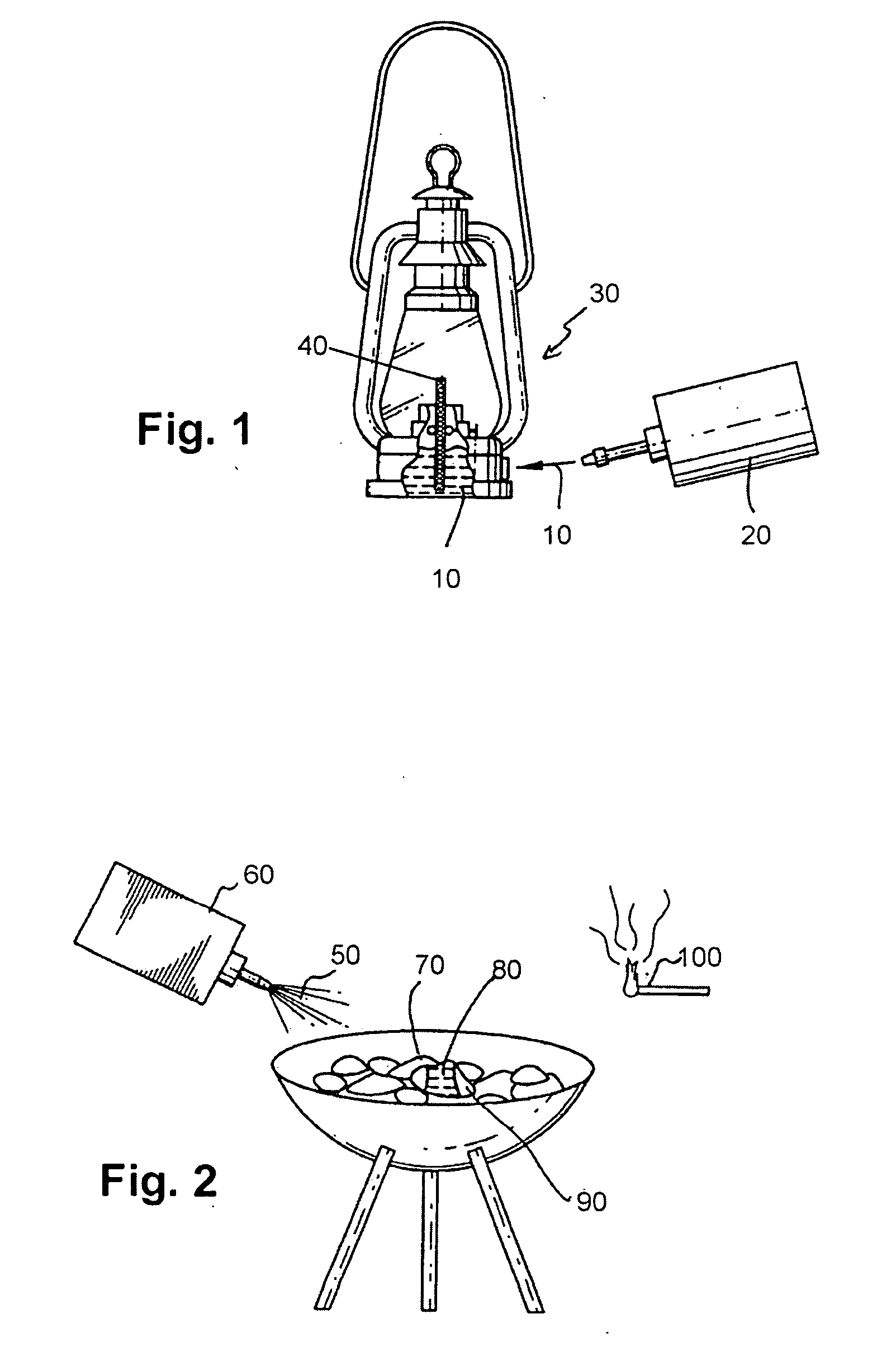
Lamp oil composition and lighter fluid composition
InactiveUS20050115145A1SaferEasy to manufactureCapillary burnersLiquid carbonaceous fuelsAlcoholLighter fuel
Lamp oil compositions including methyl palmitate, methyl stearate, myristyl alcohol, an alcohol with less than six carbons, preferably ethyl alcohol, and fragrance. Also disclosed herein are lighter fluid compositions which include methyl laurate, methyl stearate, ethyl alcohol and fragrance.
Owner:LUMETIQUE
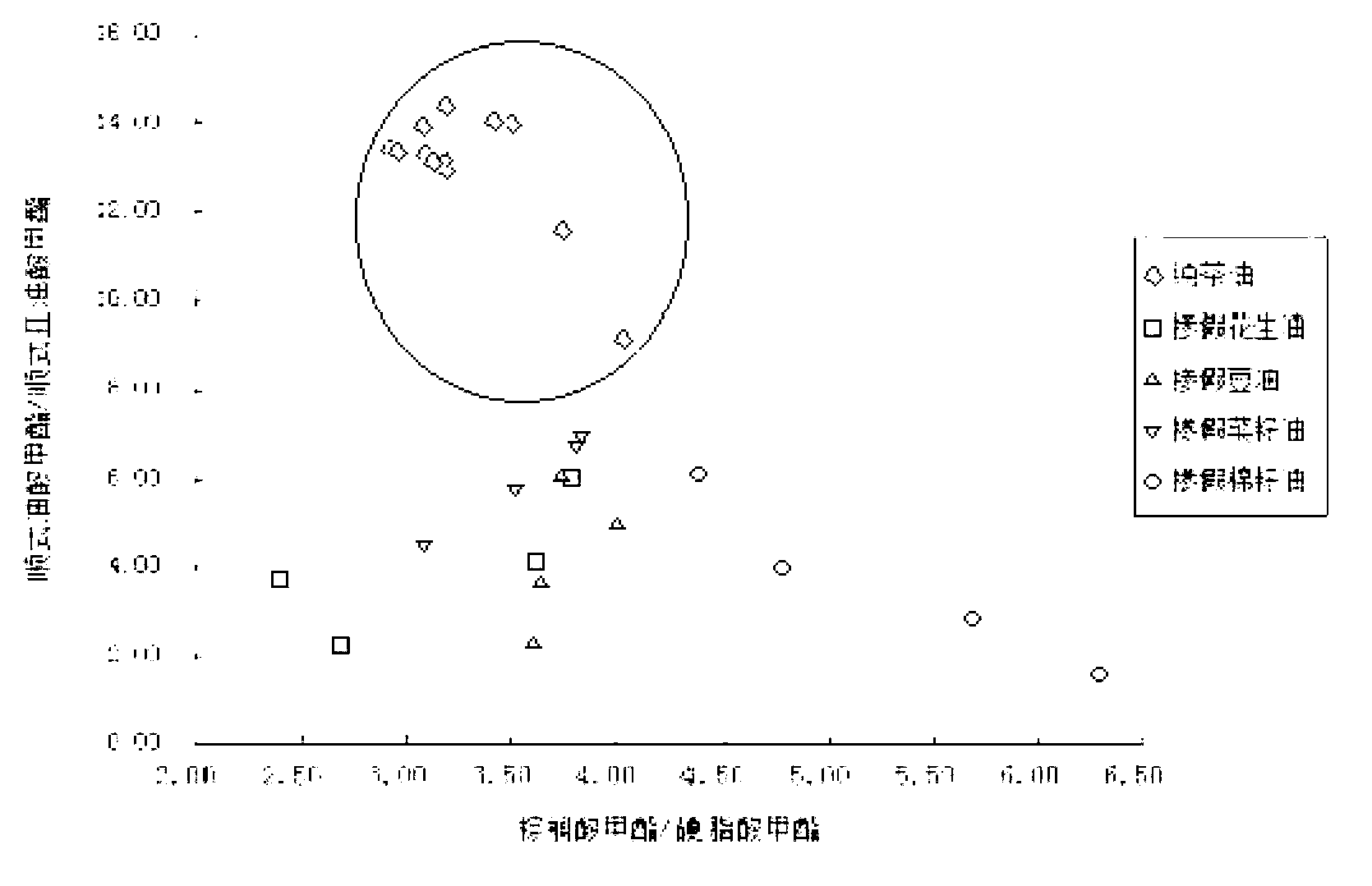
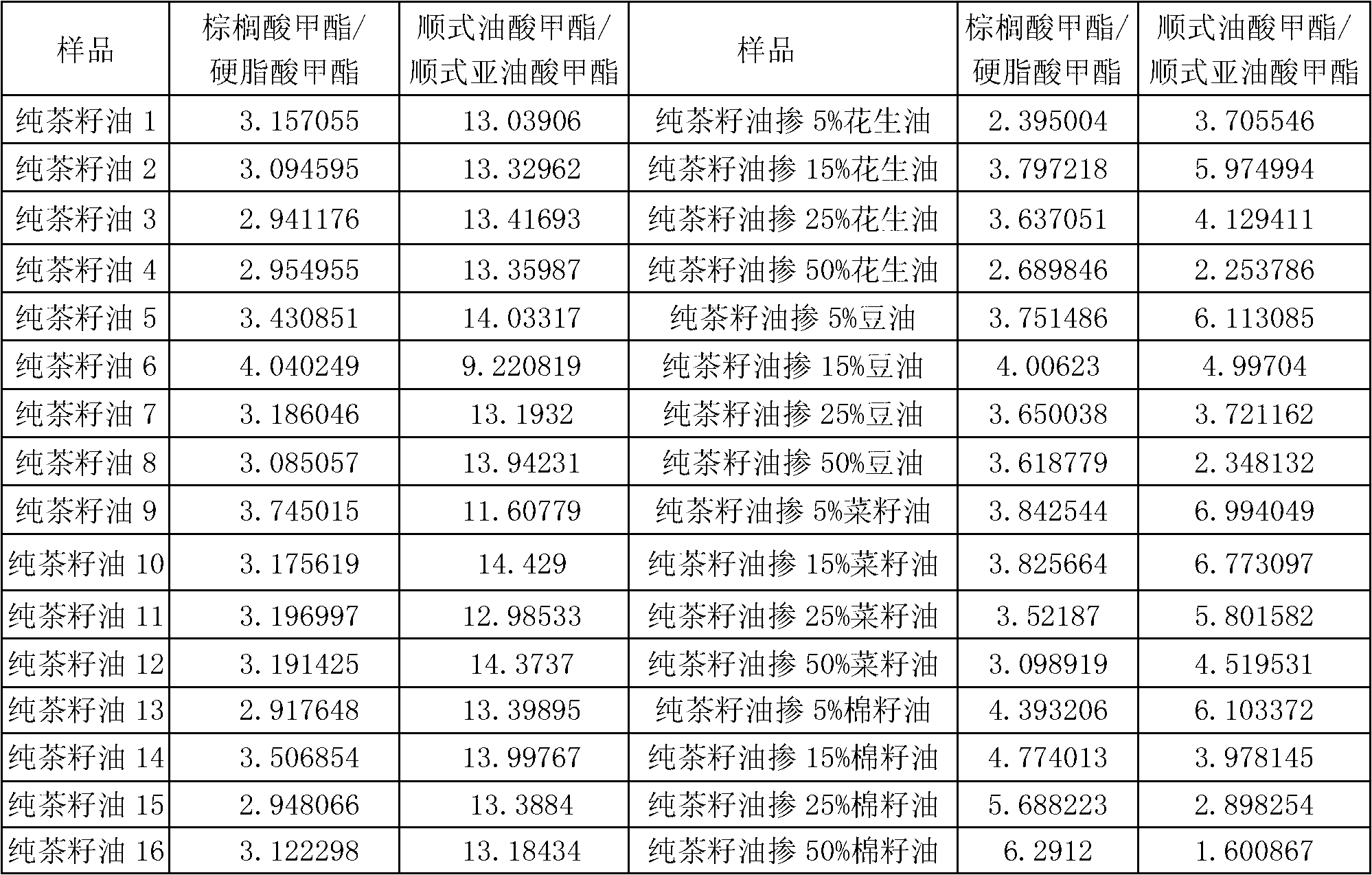
Detection method for tea seed oil adulteration based on ratio of main fatty acids
A detection method for tea seed oil adulteration based on ratio of main fatty acids obtains the contents of main fatty acids in pure tea seed oil and adulterated tea seed oil samples by applying gas chromatography and mass spectrometry technology (GC-MS technology), and intuitively presents the distribution characteristic of fatty acids of grease-adulterated pure tea oil by adopting a two-dimensional diagram of methyl cis-oleate / methyl cis-linoleate and methyl palmitate / methyl stearate, so as to qualitatively identify adulteration of tea oil.
Owner:HUNAN AGRICULTURAL UNIV
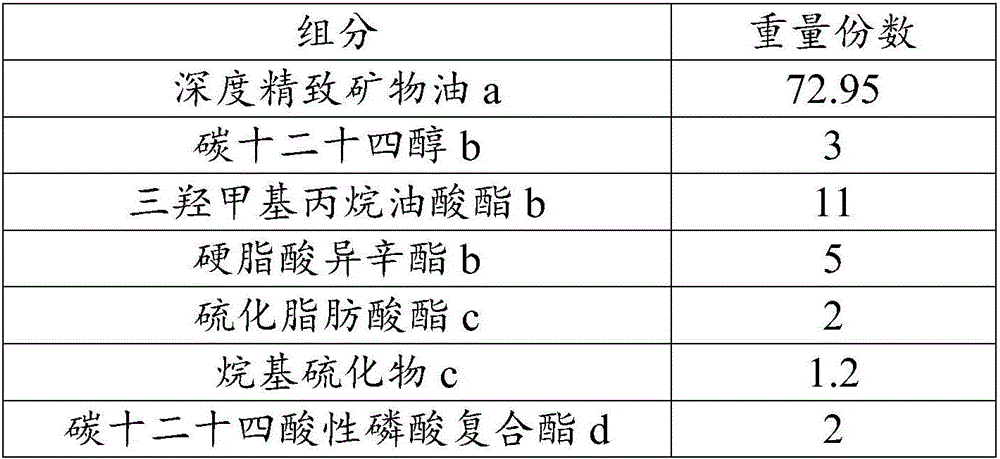
High-lubricating rolling oil composition and application thereof
ActiveCN106318568AImprove the lubrication effectMeet lubrication needsLubricant compositionPhosphateButylated hydroxytoluene
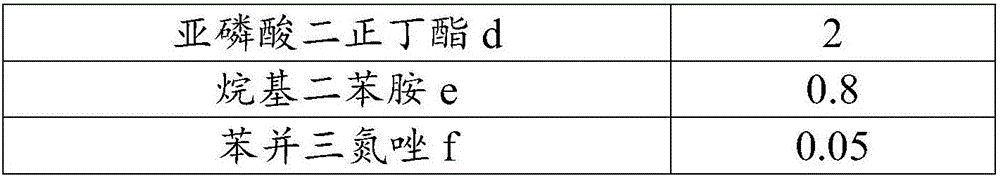
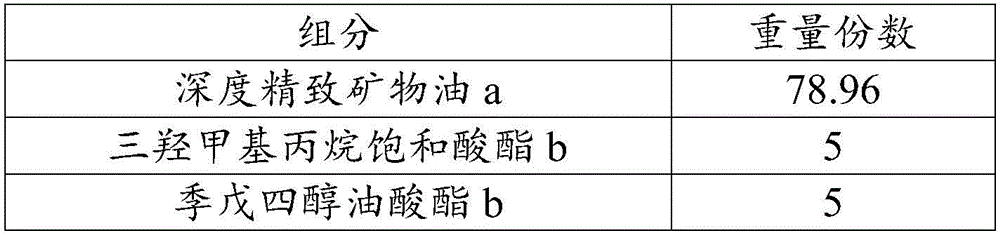
The invention relates to a high-lubricating rolling oil composition and application thereof. The composition is prepared from 40-90 parts of highly refined mineral oil, 10-30 parts of carbon dodecanol tetradecyl alcohol, isotridecyl alcohol, n-octanol, pentaerythritol oleate, trimethylolpropane trioleate, diisooctyl phthalate, 2-ethylhexyl palmitate, isooctyl stearate, methyl palmitate or butyl stearate, 1-5 parts of ZDTP, octadecyl zinc dithiophosphate, sulfurized olefin, sulfide aliphatic acid ester, sulfurized animal oil, alkyl sulfide or sulfur phosphorus molybdenum, 0.5-5 parts of amine thiophosphate, isooctyl acidic phosphate octadecylamine, tricresyl phosphate, tributyl phosphate, dibutyl phosphate and carbon-12 to carbon-14 acid phosphate compound ester, 0.1-5 parts of 2,6-butylated hydroxytoluene, N-phenyl naphthylamine, thioether phenol or alkyl diphenylamine and 0.01-2 parts of 1H-benzotriazole, thiadiazole derivative and benzotriazole.
Owner:CHINA PETROLEUM & CHEM CORP
Preparation method of gypsum and clay composite phase-change energy storing material
ActiveCN104152114AHigh strengthImprove thermal performanceHeat-exchange elementsSodium BentoniteCalcite
The invention discloses a preparation method of a gypsum and clay composite phase-change energy storing material. The composite phase-change energy storing material is characterized by being prepared from a composite phase-change precursor and a gypsum cementing material, wherein the composite phase-change precursor is prepared through the steps of adsorbing an organic phase-change agent by using an inorganic porous clay material through a solution intercalation method, then, binding by using a binder, granulating and drying; and then, the product is mixed with hemi-hydrated gypsum, and the mixture is molded to prepare the gypsum and clay composite phase-change energy storing material. The phase-change agent is selected from decanoic acid, lauric acid, myristic acid, palmitic acid, stearic acid, dodecanol, tridecanol, tetradecanol, pentadecanol, methyl palmitate, methyl heptadecanoate, ethyl heptadecanoate, methyl octadecanoate and paraffin. The inorganic porous clay material is selected from attapulgite, sepiolite, montmorillonite, bentonite, diatomite, zeolite, silica, dolomite, calcite and illite. The molding binder is selected from water glass, hydroxymethyl cellulose, silica sol and aluminum sol. The prepared composite phase-change energy storing material is wide in phase change temperature range, high phase-change latent heat, favorable in mechanical property and high in thermal stability.
Owner:BEIJING UNIV OF CHEM TECH +1
Lamp oil composition and lighter fluid composition
InactiveUS7524339B2Not to damageTransportation safetyCapillary burnersLiquid carbonaceous fuelsAlcoholLighter fuel
Lamp oil compositions including methyl palmitate, methyl stearate, myristyl alcohol, an alcohol with less than six carbons, preferably ethyl alcohol, and fragrance. Also disclosed herein are lighter fluid compositions which include methyl laurate, methyl stearate, ethyl alcohol and fragrance.
Owner:LUMETIQUE
Serum combination marker for evaluating gliclazide applicability of type 2 diabetes mellitus and detection kit thereof
ActiveCN109682909AHigh sensitivityGood repeatabilityComponent separationMetaboliteSmall molecule metabolism
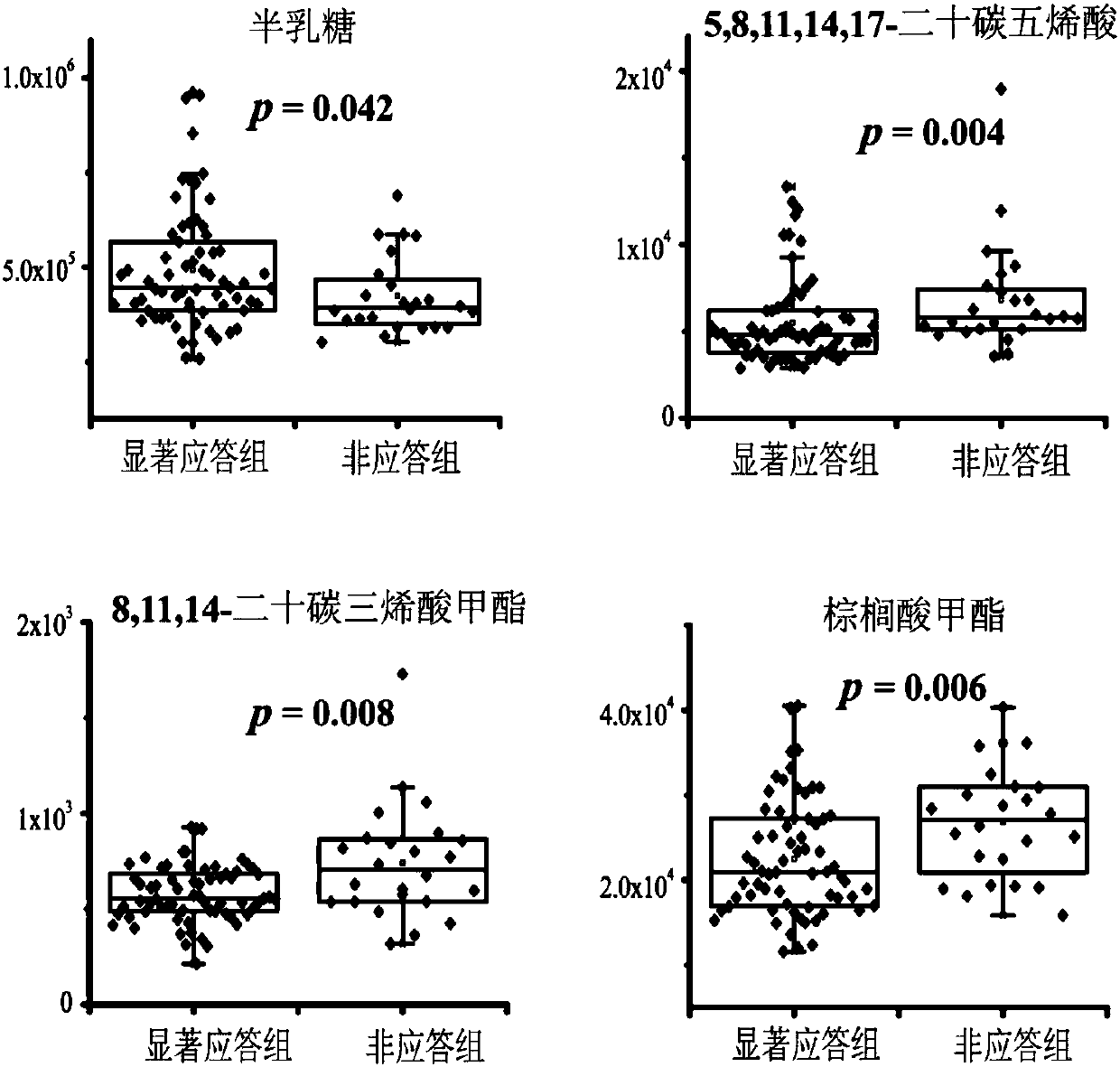
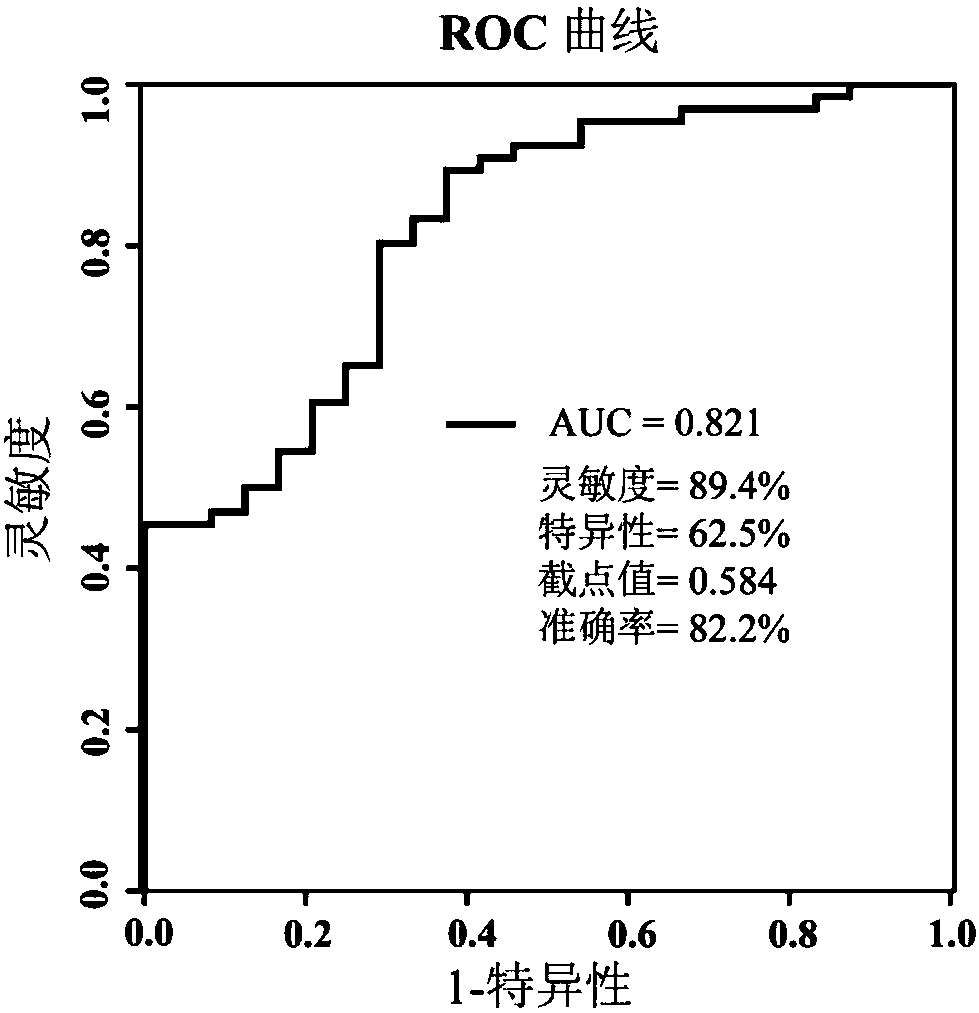
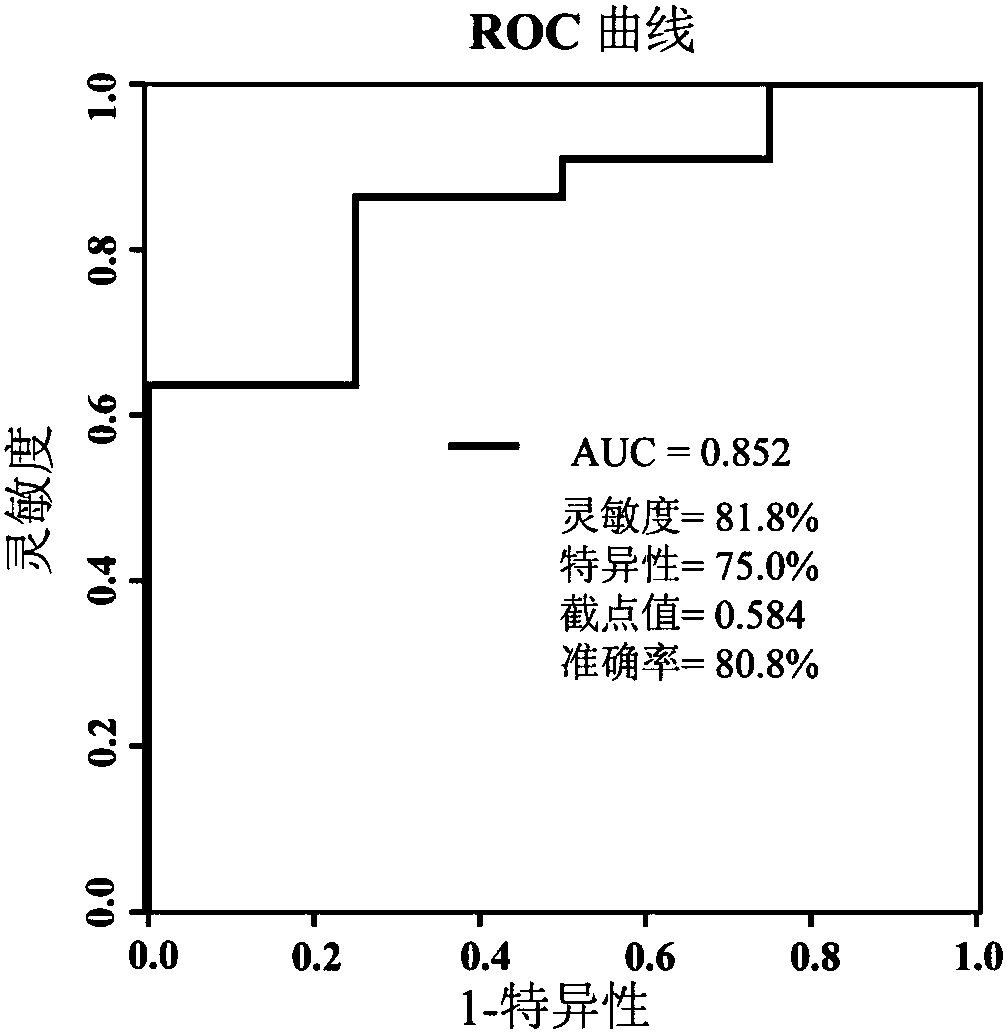
The invention relates to a serum combination marker for evaluating the gliclazide applicability of type 2 diabetes mellitus and a detection kit thereof, in particular to novel application of small-molecule metabolites, namely galactose, 5,8,11,14,17-eicosapentaenoic acid, 8,11,14-epoxyeicosatrienoic acid methyl ester and methyl palmitate, in serum samples in preparing the kit used for evaluating the gliclazide applicability of subjects as combination markers. The invention further relates to a kit for detecting patients remarkably responding to gliclazide in the subjects. By detecting relativeconcentrations of the combination markers in the serum samples of the subjects, the variables of the combination markers are calculated on the basis of a binary logic regression equation, and then onthe basis of determined section values, whether or not the subjects are suitable for gliclazide treatment is judged. The kit can achieve high-sensitivity high-efficiency detection of the small-molecule metabolites, and has the advantages of being low in detection cost, good in repeatability and high in diagnosis sensitivity.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Preparation method of clay-based composite phase-change energy storage deicing (snow melting) material
The invention discloses a preparation method of a clay-based composite phase-change energy storage deicing (snow melting) material. The preparation method is characterized in that a composite phase-change energy storage material is prepared from a low-temperature organic phase-change material and a clay material, wherein the low-temperature organic phase-change material is prepared by single-element or multi-element mixing of various organic phase-change materials and is then physically compounded with the treated clay to prepare the clay-based composite phase-change energy storage deicing (snow melting) material. The low-temperature organic phase-change material is selected from polyethylene glycol-300, polyethylene glycol-400, polyethylene glycol-500, polyethylene glycol-600, decyl alcohol, undecanol, dodecanol, heptylic acid, octanoic acid, nonanoic acid, capric acid, methyl laurate, methyl myristate, methyl palmitate, tetradecane, pentadecane, hexadecane, heptadecane, octodecane, nonadecane and the like. The clay material is selected from kaolinite, montmorillonite, bentonite, vermiculite, halloysite, sepiolite, attapulgite, perlite, diatomite, rectorite, zeolite, silicon dioxide, dolomite, calcite and illite. The prepared composite phase-change energy storage deicing (snow melting) material is proper in phase-change temperature (0-5 DEG C), high in phase-change latent heat and high in heat stability.
Owner:CENT SOUTH UNIV
High-stability environment-friendly carbon nanotube water dispersing liquid and preparation method thereof
The invention discloses a high-stability environment-friendly carbon nanotube water dispersing liquid and a preparation method thereof. The method comprises the steps: according to the mass parts, mixing 1-5 parts of carbon nanotubes, 0.5-20 parts of lignosulfonate and 0.1-5 parts of deionized water; and carrying out grinding treatment, adding water to dilute, adjusting the pH to 6-8, adding a stabilizer, and carrying out ultrasonic dispersion to obtain the stably dispersed carbon nanotube water dispersing liquid. The stabilizer is one or more of triton X-100, sodium dodecyl sulfate, sodium dodecyl benzene sulfonate, dodecyl sulfobetain and sodium methyl palmitate sulfonate. The high-stability environment-friendly carbon nanotube water dispersing liquid has the advantages of good dispersion effect of carbon nanotubes, high stability, high concentration, short technological process and preparation period, environmental friendliness and good compatibility with water-based resin.
Owner:SOUTH CHINA UNIV OF TECH
Gas chromatography method for qualitatively and quantitatively detecting soya fatty acid components
ActiveCN105044229AImprove extraction efficiencyImprove accuracyComponent separationGas phaseMethyl oleate
The invention relates to a gas chromatography method for qualitatively and quantitatively detecting soya fatty acid components. The gas chromatography method is characterized in that fatty acids in soya seeds are extracted by adopting methods of heating and methylating and detected by adopting a gas chromatography, and the actual content of the fatty acid components in the soya seeds can be accurately detected according to a standard curve and a regression equation of standard samples of five fatty acid methyl esters (methyl palmitate, methyl stearate, methyl oleate, methyl linoleate and methyl linolenate). The method is applicable to qualitative and quantitative analysis of the fatty acid components of the soya seeds, is simple and convenient to operate, easy to control and easy to popularize, can be used for detecting relative percentage content of the five fatty acid components and accurately calculating the absolute content of each fatty acid component in the soya seeds, and has an important meaning in soya fatty acid detecting and breeding.
Owner:INST OF CROP SCI CHINESE ACAD OF AGRI SCI
Preparation method of vitamin A palmitate
Owner:SICHUAN HAISCO PHARMA CO LTD
Intra-tower pump suction type high vacuum distillation method and device for precise separation of C16-C22 fatty acids
ActiveCN105695104AIncrease the compression ratioFast pumping speedOrganic compound preparationFatty acids production/refiningOil and greaseMethyl linoleate
The invention discloses an intra-tower pump suction type high vacuum distillation method and device for precise separation of C16-C22 fatty acids. The high vacuum distillation method comprises the following steps: firstly taking heat sensitive materials with a high additional value, such as animal and vegetable oil, as raw materials, adopting plasma liquid and the like as an esterification and transesterification catalyst, and obtaining mixture of multiple fatty acid methyl esters with high yield in one step; secondly, in a distillation tower with almost zero pressure drop, carrying out high vacuum distillation operation on the formed multiple fatty acid methyl esters through catalysis; and finally separating and purifying, so that single fatty acid methyl esters such as timnodonic acid methyl ester, docosahexoenoic acid methyl ester, methyl palmitate, methyl stearate, methyl oleate and methyl linoleate with the mass fraction more than 98% respectively are obtained. The whole technological method is simple and clear, the operation is simple, the equipment cost is low, the energy is saved, and the enlargement can be easily realized.
Owner:TIANJIN UNIV
Production method of sucrose ester and use thereof in preparation of special antioxidant for oil
ActiveCN102533454AOptimize and simplify the purification processShort processFatty acid esterificationEdible oils/fatsChemical synthesisSucrose
The invention relates to a production method of sucrose ester with low HLB (Hydrophile-Lipophile Balance) and use thereof in preparation of a special antioxidant for oil. The production method of the invention comprises the following steps: (1) adding tea seed oil, methanol and concentrated sulfuric acid to synthesize tea seed oil fatty acid methyl ester; (2) adding KOH solution, stearic acid, cane sugar, a catalyst and methyl palmitate and mixing with the tea seed oil fatty acid methyl ester, and preparing sucrose fatty acid ester with high degree of substitution by an ester exchange method;adding absolute ethyl alcohol to reflow so as to separate and purify the sucrose fatty acid ester, completely removing the catalyst and the un-reacted raw materials by petroleum ether, and removing the petroleum ether to obtain the available sucrose ester. The sucrose ester with low HLB value prepared by the invention is compounded with vitamin E, carnosic acid or tea polyphenol, and can be used for producing a natural oil antioxidant with excellent production property and replacing a chemical synthesis oil antioxidant to be forbidden.
Owner:LIUZHOU GAOTONG FOOD CHEM
Wheat bran oil production technology using subcritical butane extraction
InactiveCN107338106ASimple processHigh extraction rateFatty-oils/fats productionMethyl linoleateDistillation
The invention discloses a wheat bran oil production technology using subcritical butane extraction, and belongs to the field of food engineering. Wheat bran undergoes subcritical butane extraction multiple times, and the obtained extract liquid undergoes reduced pressure distillation to remove an extractant to obtain the wheat bran oil containing methyl palmitate, methyl linoleate and methyl oleate. The wheat bran is cyclically extracted by adopting a subcritical butane technology, and technologic optimization researches of factors affecting the yield of the wheat bran oil are carried out by adopting Box-Benhnken center combination response surface test design in order to obtain optimum technologic parameters; and a gas chromatograph-mass spectrometer (GC-MS) is used to analyze the fatty acid components of the wheat bran oil obtained through the subcritical extraction in order to provide reference for the development and the utilization of wheat bran resources. The wheat bran oil production technology is simple, allows the extraction rate to reach up to 86.92%, is suitable for industrial application, and provides a new way for the further utilization of the wheat bran.
Owner:QILU UNIV OF TECH
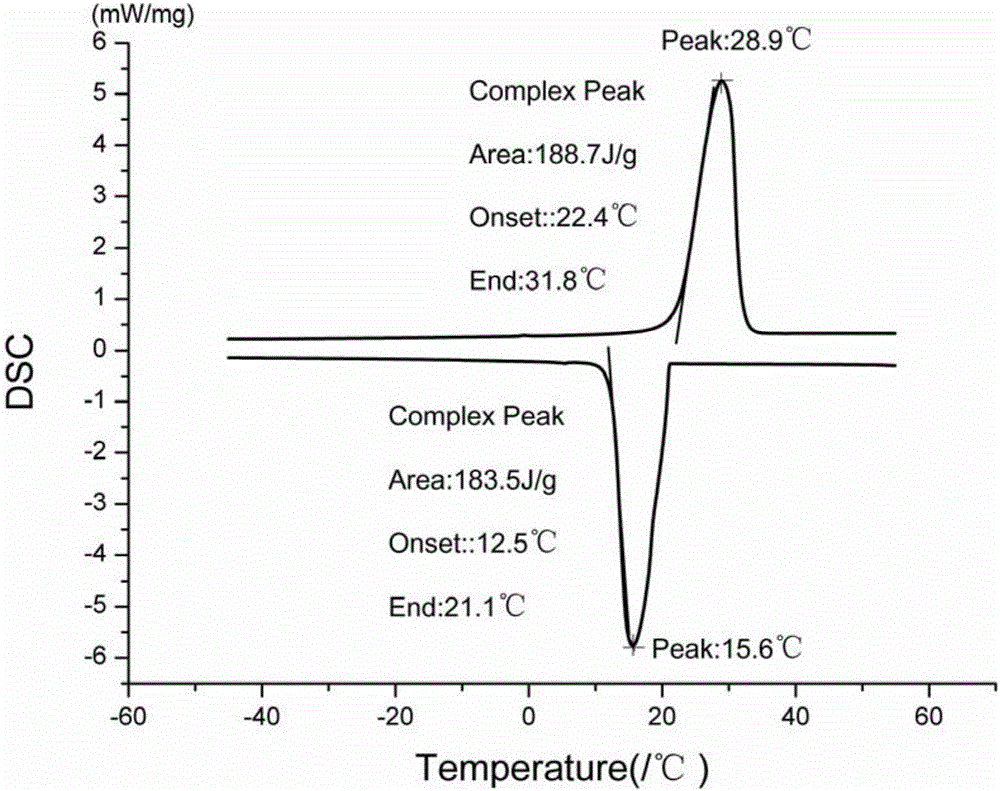
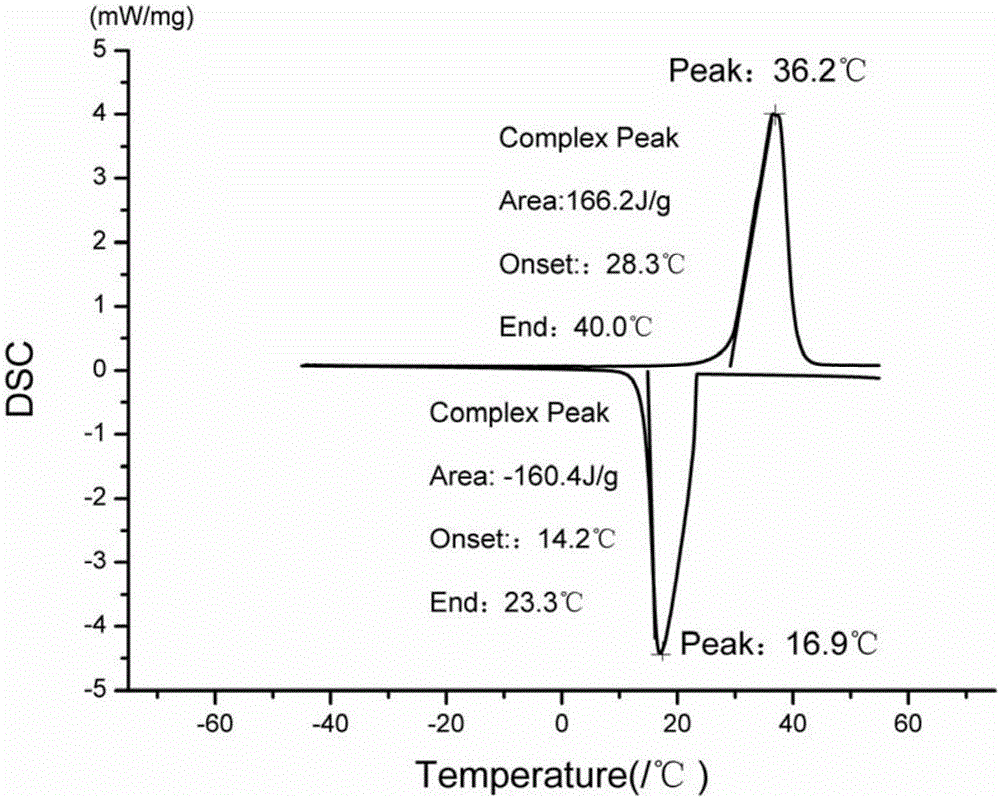
Methyl hexadecanoate-methyl stearate composite phase-change energy-storage material and preparation method thereof
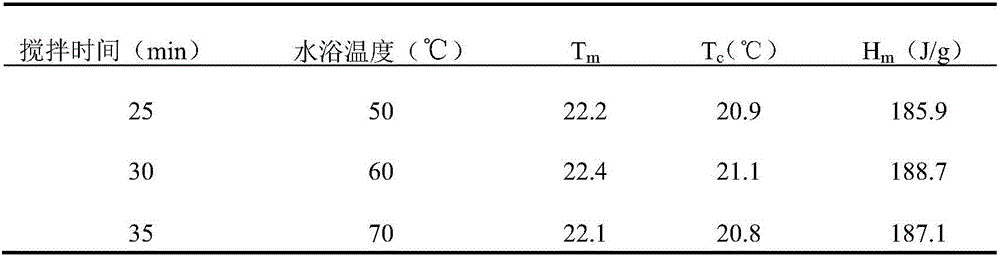
The invention discloses a preparation method of a methyl hexadecanoate-methyl stearate composite phase-change energy-storage material. The preparation method of the methyl hexadecanoate-methyl stearate composite phase-change energy-storage material comprises the following steps that methyl hexadecanoate and methyl stearate are added to a reaction container according to the mass ratio of one fourth to four, the mixture is heated in a thermostatic water bath and stirred for 25-35 min, the mixture is then cooled to the indoor temperature, and then the methyl hexadecanoate-methyl stearate composite phase-change energy-storage material is obtained, wherein the temperature of the thermostatic water bath is set to be 50-70 DEG C. According to the preparation method of the methyl hexadecanoate-methyl stearate composite phase-change energy-storage material, the phase change material has the advantages that the phase change latent heat is high, the volume change in the phase change process is small, the surface temperature fluctuation is low, the phase change material is well fused with a building material and low in cost, and the phase change temperature of the phase change material is closest to the indoor and outdoor optimum temperatures, and the advantages of the phase change material are utilized for synthesis of the composite phase-change energy-storage material; and the phase change temperature of the composite phase-change energy-storage material is obviously lower than a single phase change material, the comfortable state that the indoor temperature gradient is lowered to be smaller than 5 DEG C can be achieved, the service efficiency of an air conditioner is improved, and energy is saved.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
Temperature sensitive ink
The invention discloses temperature sensitive ink, which is suitable for offset printing or flexography on a rotary press. The temperature sensitive ink consists of following three parts by weight percent: totally about 48 percent of petroleum resin (large amount), paraffin (small amount) and pigment (trace amount), totally about 41 percent of methyl stearate (large amount), methyl palmitate (small amount) and 2, 6-ditertiary butyl-p-hydroxytoluene (trace amount), and totally about 11 percent of methylbenzene and dimethylbenzene. If necessary, about 3 percent of fluorescent powder can be added. By adopting the special formula, the ink is suitable for offset printing or flexography on the rotary press on which common temperature sensitive ink cannot be used. Moreover, the ink has the temperature sensitive color changing function or not only the temperature sensitive color changing function but also the fluorescence detection function.
Owner:吴月梅
Deoxyfructosazine containing additive agent for fragrance compensation for low-coke tar cigarette
ActiveCN101317693AIncrease the amount of aromaImprove coordinationTobacco preparationNon-fibrous pulp additionCyclopentenePropanoic acid
The invention discloses an additive containing deoxyfructosazine for applying fragrance compensation to low-tar cigarettes, which is characterized in that the additive comprises the following raw materials: 0.001 to 5 percent of deoxyfructosazine, 0 to 1 percent of furfural, 0.1 to 0.5 percent of 5-methyl-furfural, 0.1 to 1 percent of 3-ethyl-2-hydroxyl-2-cyclopentene-1-ketone, 0.005 to 5 percent of Peru concrete, 0.1 to 1 percent of methyl palmitate, 0.1 to 1 percent of damascenone, 0.1 to 2 percent of Megastigmatrieno, 0.1 to 3 percent of cocoa powder tincture, 0 to 1 percent of propionic acid, and 82 to 99 percent of ethanol containing 50 percent of water in weight percentages; the additive with a weight percentage of 0.1 to 5 percent is used for aromatizing tobacco leaves or coil paper of dark color, and can obviously improve the fragrance volume of high-tar and low-tar cigarettes and also has good compatibility with tobacco flavor, decreases stimulation and improves the comfort degree of nonnasality.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Lubricating agent composition for white alloy cold drawing machining
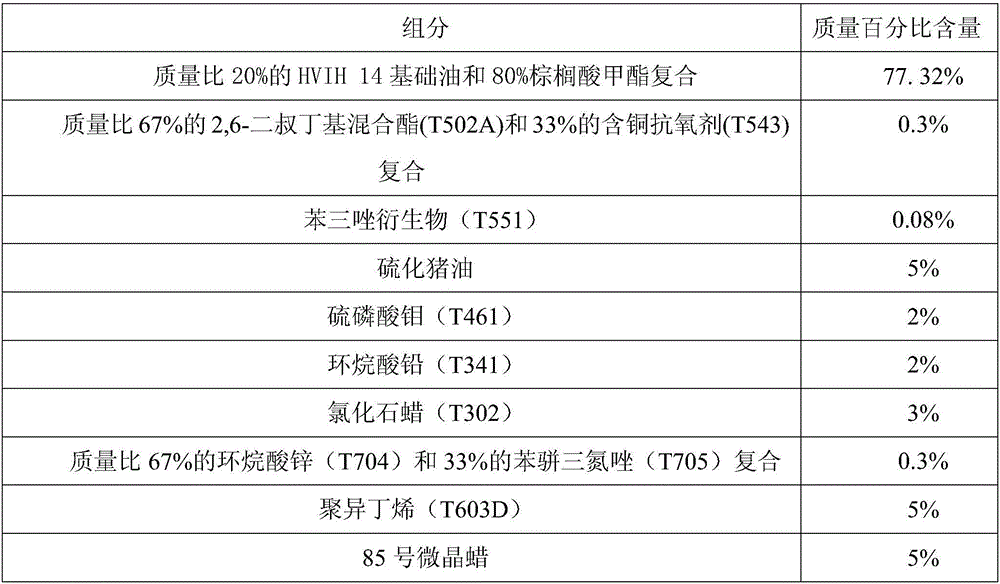
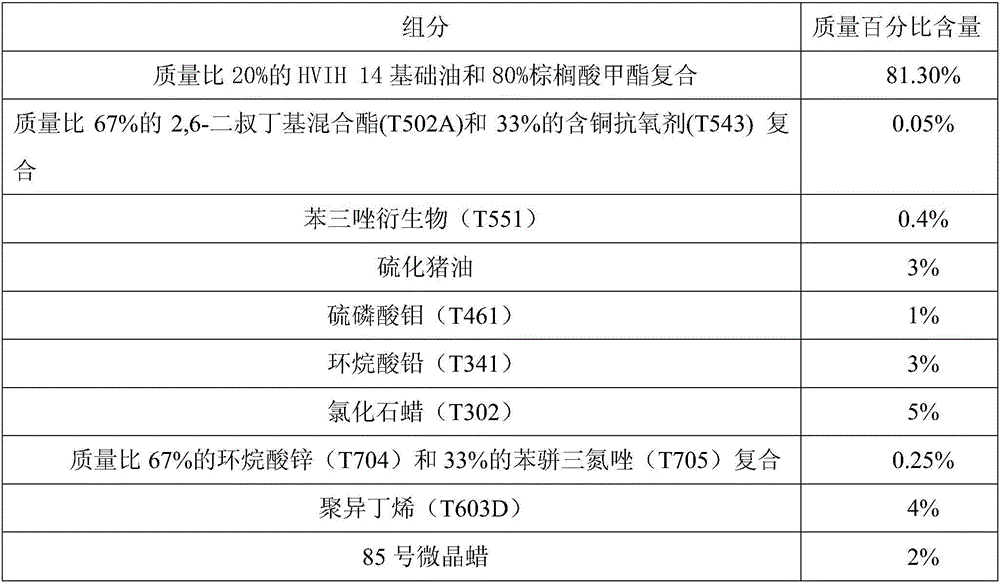
The invention discloses a lubricating agent composition for white alloy cold drawing machining. A compound of HVIH14 and methyl palmitate is adopted as basic oil and cooperates with various composite additives. The lubricating agent composition includes antioxidant, a metal passivator, an oiliness agent, antiwear agent, an extreme pressure agent, a friction improver, a viscosity index improver, a filling agent, antirust agent and others. The composition has the advantages that the number of machined workpieces and molds needing to be rubbed, lubricated and cooled is reduced, power consumption is lowered, and deformation is uniform; the service life of a tool is prolonged, and abrasion of workpiece materials is controlled; the quality of the surface of machined metal is improved, and the tolerance of the surface of the machined metal is reduced; temperature is controlled, and machining deformation is reduced; new and old surfaces of the machined metal are kept oxidized or passivated; using is safe, and toxins do not exist; residues do not affect machining and operating, and cleaning is convenient.
Owner:GUANGXI UNIV
Special biological grease for powder emulsion explosive
ActiveCN103086811ASatisfy the emulsificationSatisfy emulsification stabilityNon-explosive/non-thermic compositionsWaxEmulsion explosive
The invention discloses special biological grease for a powder emulsion explosive. The special grease mainly comprises a raw material and an additive, wherein the main raw material is formed by mixing the following components in percentage by mass: 20 to 60% of vegetable wax, 10 to 30% of stearic acid and 0 to 20% of methyl palmitate; and the sum of the mixed raw materials and the additive is 100%. By adopting the special grease, the quality fluctuation due to more components added is avoided, and the problems due to complicated operation process, high working strength of workers and poor production environment can be solved.
Owner:福建达安能源实业有限责任公司
Combined remediation method of polycyclic aromatic hydrocarbon polluted soil
ActiveCN104492795ASimple stepsReduce PAH contentContaminated soil reclamationSubtilisinPolycyclic aromatic hydrocarbon
The invention belongs to the field of soil remediation, and discloses a combined restoration method of polycyclic aromatic hydrocarbon polluted soil, which comprises the following steps: (1) putting polycyclic aromatic hydrocarbon polluted soil in a bioreactor, adding water at room temperature, and stirring; (2) adding 5-30U of subtilisin to every gram soil of into the bioreactor, and stirring at 30 rpm; (3) while stirring, adding sodium palmitate and methyl palmitate into the bioreactor, and continuing stirring, wherein the sodium palmitate accounts for 3.5-5 wt% of the soil, and the methyl palmitate accounts for 0.8-1.6 wt% of the soil; and (4) adding cotton straw powder into the bioreactor (the cotton straw powder accounts for 1.5-4.5 wt%), continuing stirring for 2 hours, standing, separating the soil from water, and drying to obtain the remediated soil.
Owner:JIANGSU GAIYA ENVIRONMENTAL SCI & TECH CO LTD
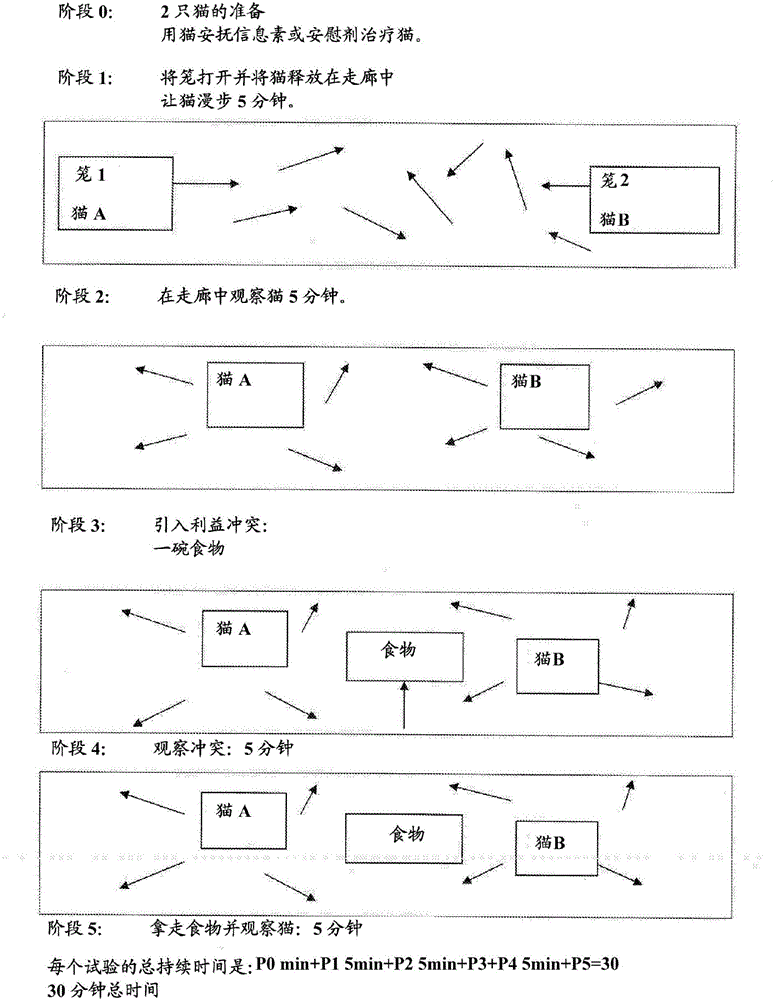
Cat appeasing pheromone
The present invention relates to a semiochemical composition comprising methyl palmitate, methyl linoleate, methyl oleate methyl stearate, methyl laurate, methyl myristate, salts thereof, derivatives thereof, isomers thereof and / or structural analogues thereof that have an appeasing effect in cats and / or also effects social facilitation in cats, and an acceptable vehicle. Solutions such as spot-on formulations of long duration are also encompassed. Methods to effect appeasing in a cat and / or social facilitation in cats are also disclosed.
Owner:INST OF INFORMATION CHEM & APPLIED ANIMAL BEHAVIOR
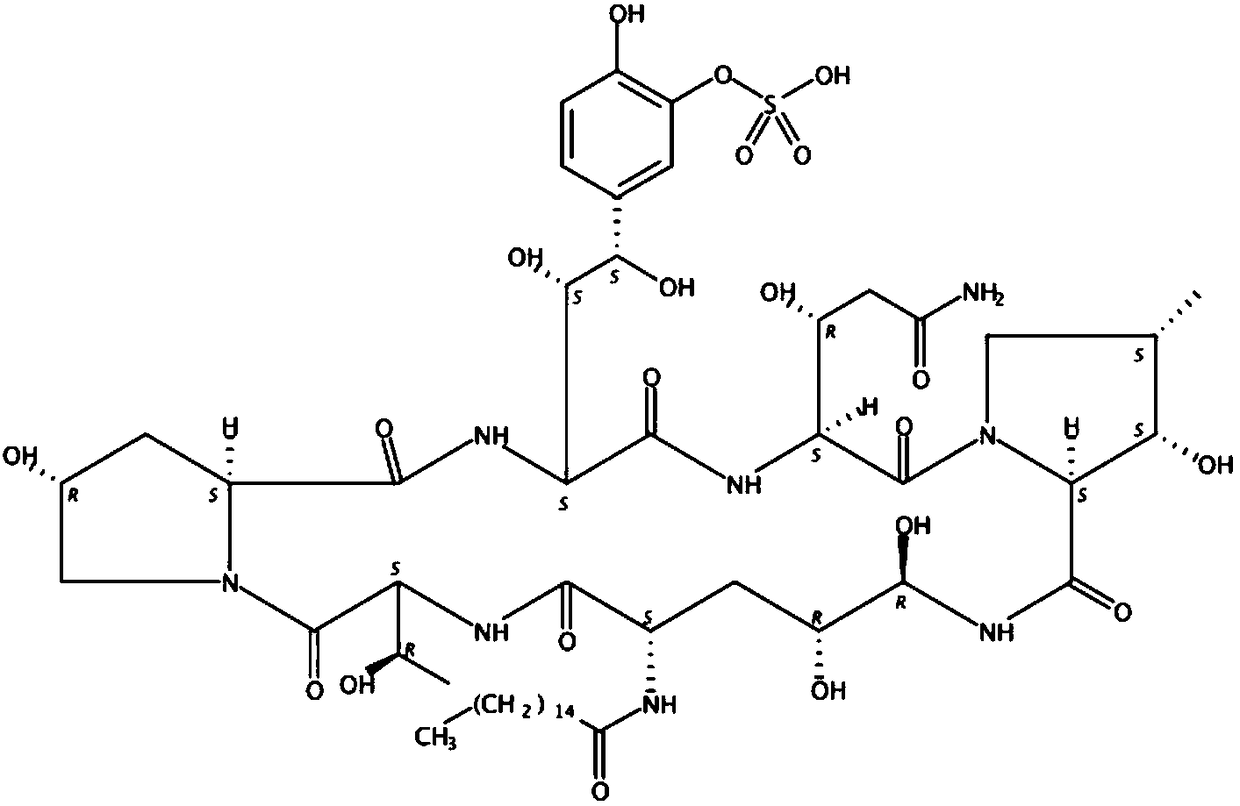
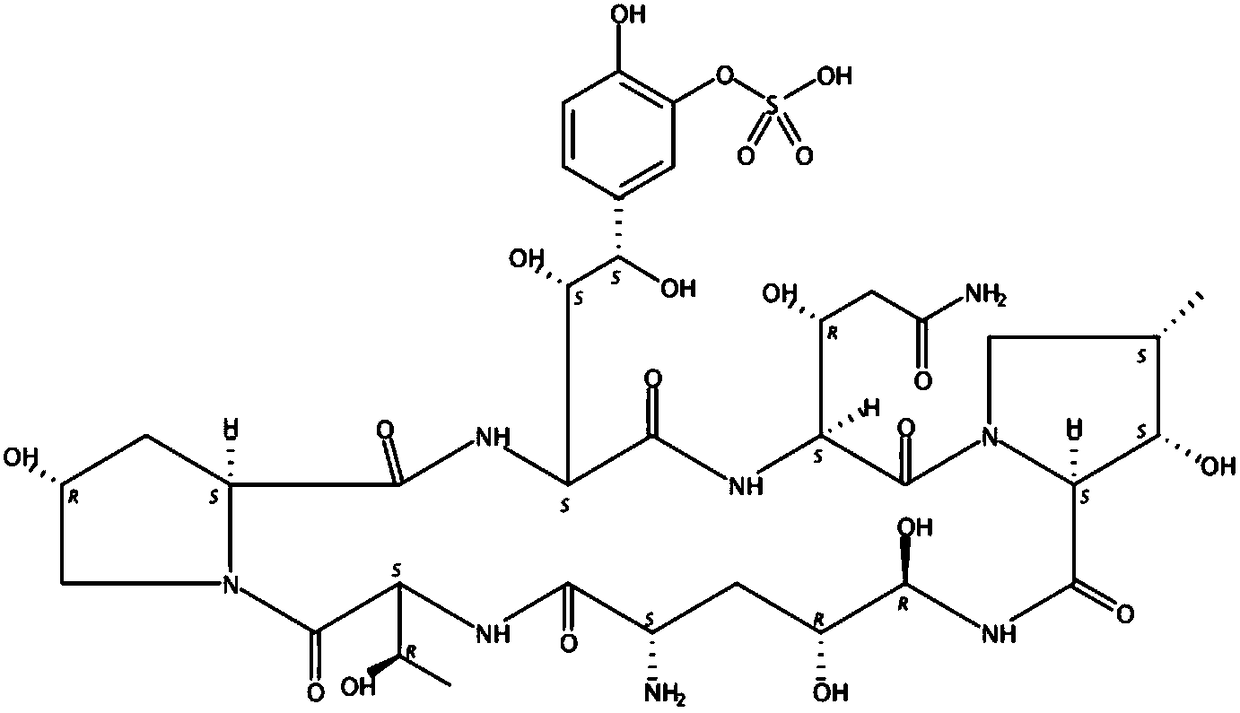
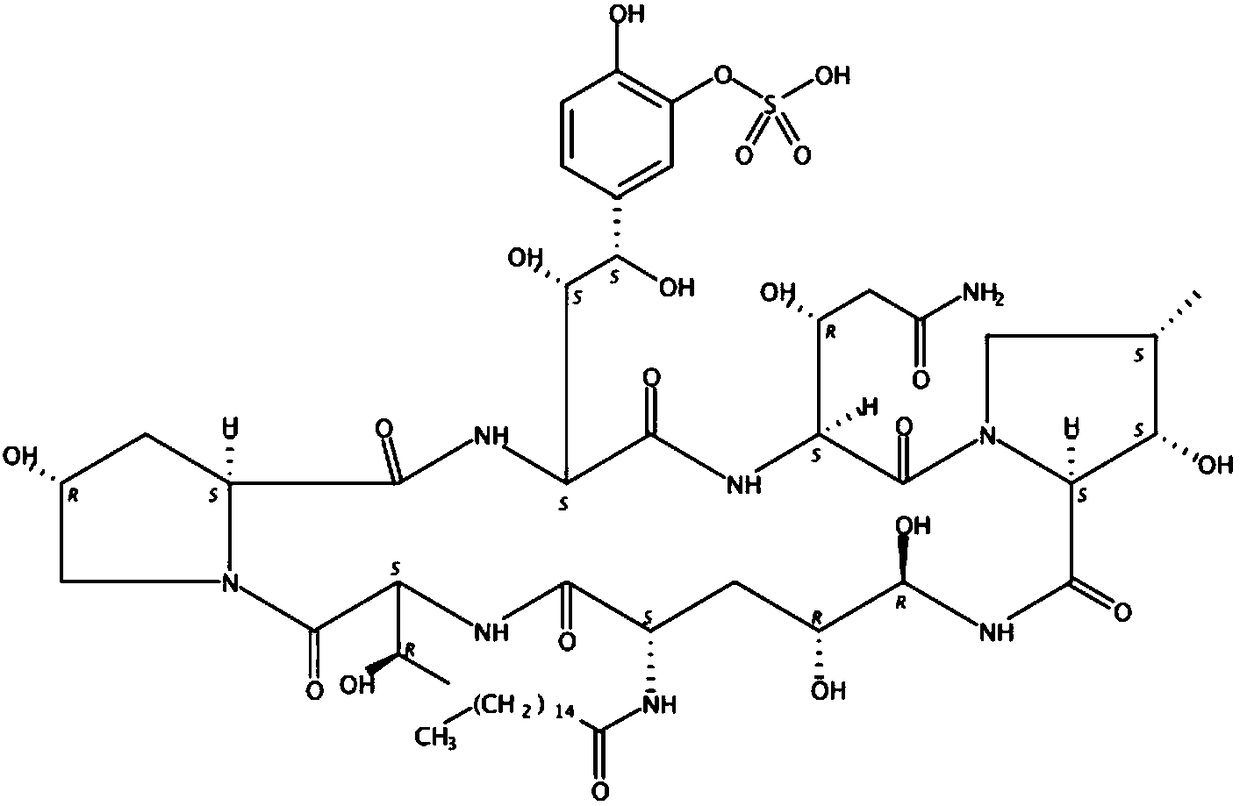
Novel preparation method of micafungin sodium precursor
InactiveCN108753880APromote biosynthesisImprove fermentation yieldMicroorganism based processesPeptidesCross-linkFermentation broth
The invention discloses a preparation method of FR179642. The method comprises the following steps: 1) preparing a medium for fermenting FR901379; 2) inoculating a fermentation medium with a bacterialstrain Coleophoma empetri seed culture solution for fermentation; 3) supplementing a mixture of methyl palmitate and dimethicone to a fermentation broth after fermentation culture for 24-72 h; 4) finishing fermentation when the concentration of FR901379 no longer increases after fermentation for 160 h; 5) enabling FR901379 obtained in the previous step to be subjected to a reaction with a deacylase cross-linked enzyme polymer to be converted into FR179642.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
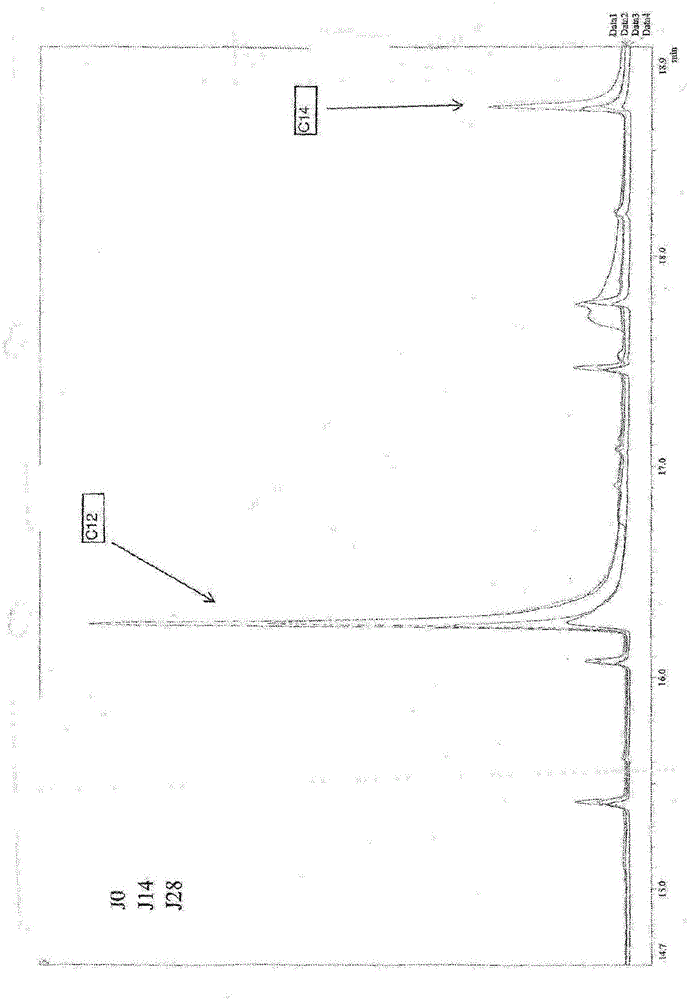
Improved method for measuring fatty acid composition of sorbitan monostearate
The invention discloses an improved method for measuring the fatty acid composition of sorbitan monostearate. The method comprises: taking a sample and placing the sample into a flask; adding a potassium hydroxide methanol solution, carrying out heating refluxing, and carrying out cooling; adding a methanol solution, carrying out heating refluxing, and carrying out cooling; adding normal hexane, and carrying out heating refluxing continuously, and carrying out cooling; adding a saturated sodium chloride solution, carrying out shaking uniformly, carrying out standing for layering, and taking supernatant fluid and carrying out anhydrous sodium sulfate drying; carrying out testing based on a gas chromatographic method; with a capillary column with polyethylene glycol as stationary liquid as achromatographic column, taking a proper amount of methyl palmitate and methyl stearate reference substances respectively and carrying out dilution to make a solution containing 1mg of methyl palmitate and 1mg of methyl stearate respectively per 1ml under conditions of keeping the feeding inlet temperature of 240 DEG C and the detector temperature of 280 DEG C; injecting the solution into a gas chromatograph and recording a chromatogram map with the separation degrees of all chromatographic peaks meeting requirements; and taking the supernatant fluid and injecting the fluid into the gas chromatograph, recording a chromatogram map, and carrying out calculation with a peak area based on an area normalization method. With the disclosed method, the error rate is low.
Owner:JIANGSU SEMPOLL PHARMA
Phosphate ore reverse flotation collecting agent capable of removing calcite
The invention relates to a phosphate ore reverse flotation collecting agent capable of removing calcite. The collecting agent is formed by compounding 80-90 % of a soaping agent with 10-20% of a composite surfactant by weight, wherein the soaping agent is formed by compounding 15% of cottonseed oil soap, 15% of soybean oil soap, 10% of rosin soap, 25% of rice bran oil soap and 35% of palm oil soap, and the composite surfactant is formed by compounding 25% of sodium diethylhexyl sulfosuccinate, 25% of sodium methyl palmitate sulfonate and 50% of diethyl phthalate. The collecting agent is suitable for the sedimentary type phosphate block rock reverse flotation foam floating process capable of removing calcite. The collecting agent has the characteristics of good selectivity and high concentrate recovery rate.
Owner:HUBEI FORBON TECH
Cat face pheromone and application thereof
PendingCN112516128AAlleviate or relieve stressful behaviorLow costNervous disorderEster active ingredientsMethyl linoleateDIMETHYL SEBACATE
The invention discloses cat face pheromone and application thereof. The cat face pheromone comprises a cat face pheromone analogue and a solvent, and the cat face pheromone analogue comprises methyl laurate, methyl myristate, methyl palmitate, methyl oleate, methyl linoleate and dimethyl sebacate. The cat face pheromone can be used for preparing a preparation for reducing and relieving anxiety andconflicts after a cat is blended into a new environment, is suitable for a scene that the surrounding environment of the cat is changed, and can ease or relieve stress behaviors of the cat in the newenvironment, such as abnormal emotion and even pathological diseases; and meanwhile, since dimethyl heptanedioate and dimethyl azelate which are relatively high in price are not adopted for the cat face pheromone analogue and the cat face pheromone, the cost of the used raw materials is relatively low, the product is suitable for mass production, the purity of the raw materials is improved, the product quality is improved, and the effect is exerted.
Owner:上海弗艾柏生物科技有限公司
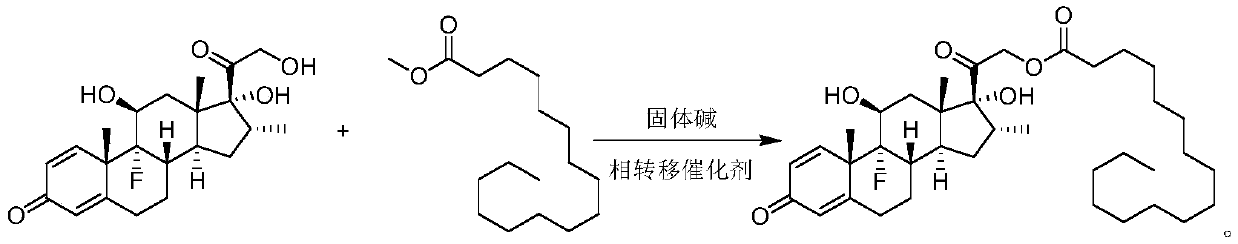
Synthesis method of dexamethasone palmitate
The invention relates to a synthesis method of dexamethasone palmitate. The method comprises the steps as follows: dexamethasone and methyl palmitate are subjected to transesterification under catalysis of solid alkali and a phase transfer catalyst to obtain a target product; a mixture of methyl palmitate and the product obtained after a solvent is removed from solid alkali and the phase transfercatalyst by evaporation is recycled. The method is mild in reaction condition, green, environmentally friendly, simple, efficient and energy-saving, raw materials are easy to obtain, the production cost is reduced, the operation environment is improved, and the method is convenient to operate, high in automation degree and particularly prone to industrialization.
Owner:JIANGSU YUANDA XIANLE PHARMA
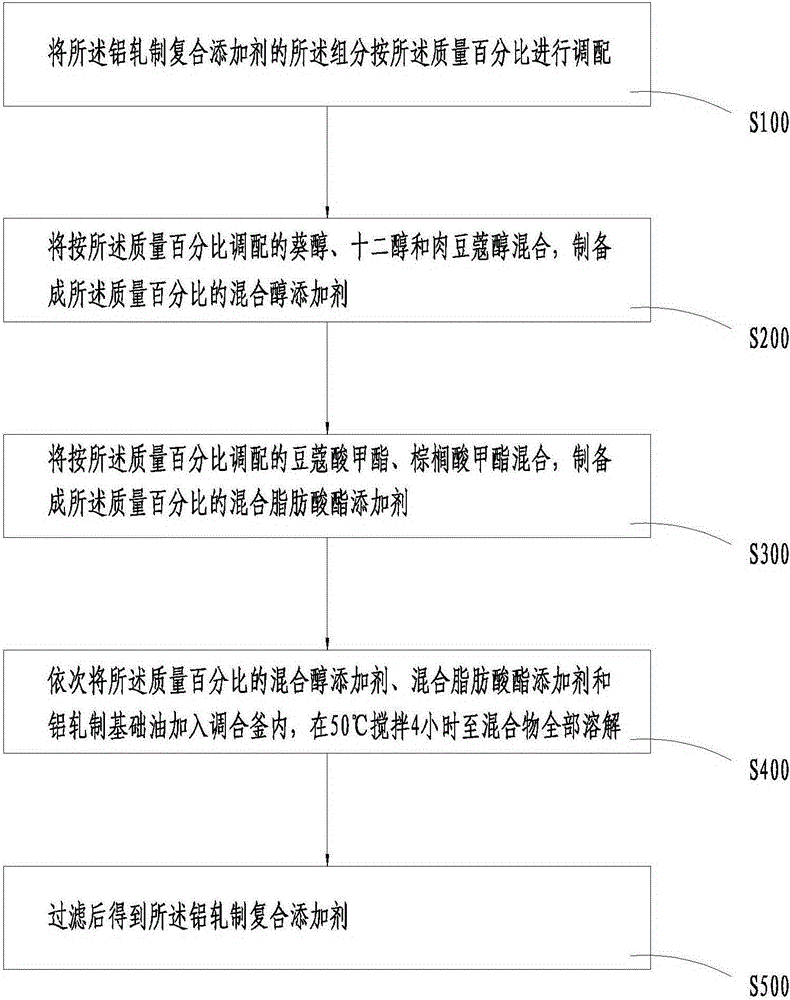
Aluminum rolling composite additive and preparation method thereof
InactiveCN105219501AGood annealing cleanlinessGuaranteed lubrication effectLubricant compositionMixed fatty acidAlcohol
The invention provides an aluminum rolling composite additive which is prepared from, by mass, 65.0-79.0% of mixed alcohol additives, 15.0-25.0% of mixed fatty alcohol acid ester additives and 6.0-12.0% of aluminum rolling base oil. The mixed alcohol additives are prepared from, by mass, 15.0-25.0% of n-decyl alcohol, 28.0-38.0% of dodecanol and 40.0-50.0% of myristyl alcohol. The mixed fatty alcohol acid ester additives are prepared from, by mass, 70.0-80.0% of tetradecanoic acid-methyl ester and 20.0-30.0% of methyl palmitate. Compared with the prior art, the aluminum rolling composite additive has the advantages of being good in annealing detergency, high in annealing product surface quality and low in annealing energy consumption.
Owner:SNTO TECH GRP
Active solvent for relieving sump oil blockage of water injection well
The invention provides an active solvent for relieving sump oil blockage of a water injection well. The active solvent is prepared by mixing fatty acid methyl ester, a penetrating agent and organic alkali, wherein the weight percent of the penetrating agent is 1-5%; the weight percent of the organic alkali is 5-20%; and the others are fatty acid methyl ester; the penetrating agent is selected from isooctyl alcohol polyoxyethylene ether-4, isooctyl alcohol sulphonated succinate or isooctyl alcohol polyoxyethylene ether phosphate-4; the organic alkali is selected from one or more of triethanolamine, triethylamine, diethylamine or dimethylamine; the fatty acid methyl ester is selected from one or more of methyl laurate, methyl palmitate, methyl oleate and methyl stearate; and the fatty acid methyl ester has strong dissolving capacity on the sump oil; the sump oil injected on an infiltration surface of the water injection well can be dissolved, and pushed away from the infiltration surface of the water injection well; tallate and methanol can be generated after the sump oil is hydrolyzed in stratum; the carried sump oil can be precipitated to advance towards the deep part of the stratum continuously. Thus, the effect of the sump oil on the infiltration surface of a near well is relieved.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA) +1
Low-temperature purification and separation process for fatty acid lower alcohol ester plasticizers based on palm oil
InactiveCN107840800ASufficient and stable sourceReduce manufacturing costOrganic compound preparationCarboxylic acid esters preparationAlcoholUnsaturated fatty acid ester
The invention discloses a low-temperature purification and separation process for fatty acid lower alcohol ester plasticizers based on palm oil. According to the process, micromolecular alcohol is used as a solvent; programmed cooling is carried out to allow methyl palmitate with a high-melting point to be precipitated through crystallization; and palmitate and unsaturated fatty acid esters in fatty acid lower alcohol esters are almost separated and purified by using the process. The process provided by the invention is low in energy consumption, simple in process, good in operability and friendly to environment. Products obtained through multiple separation operations are high in purity and widely applicable to industrial fields like plastics and rubber, are good substitutes for petroleumproducts and have good development prospects.
Owner:北京林氏精化新材料有限公司
Processing method of flower fragrance-type black tea cake
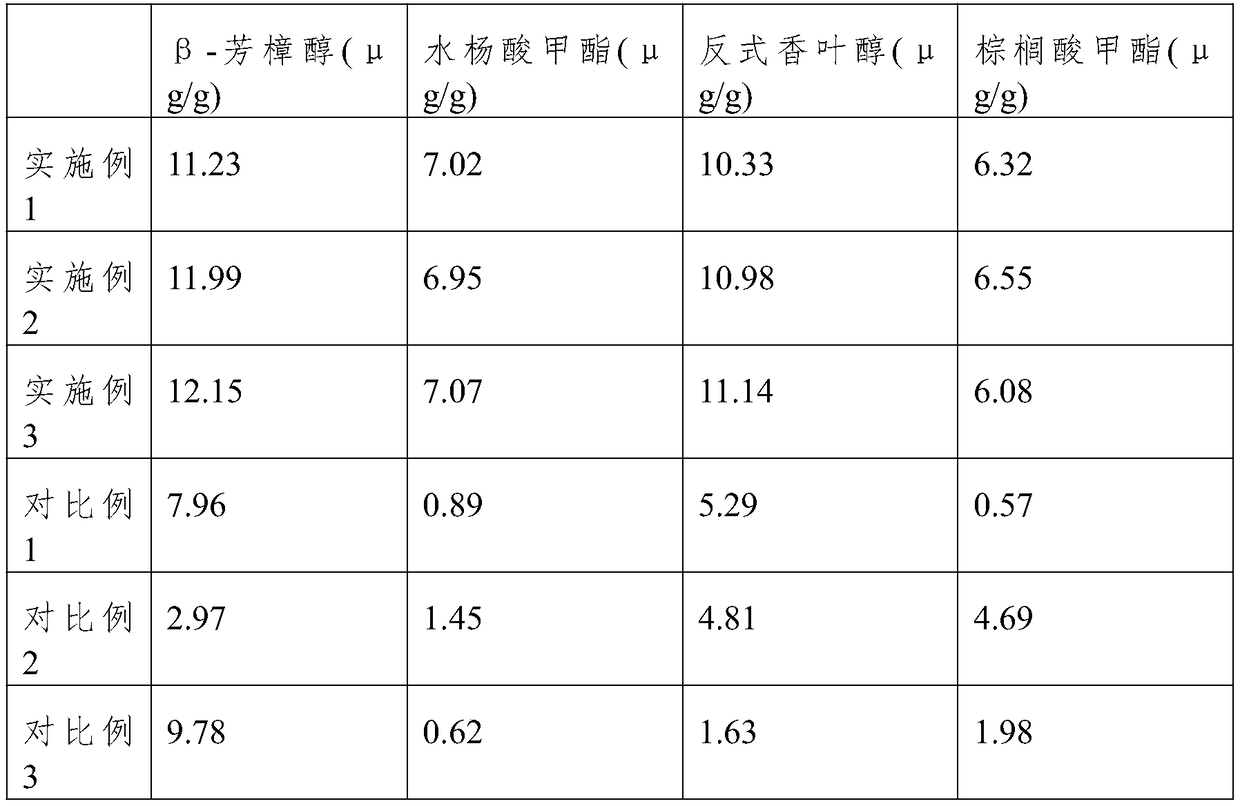
ActiveCN108902375AEffective damageImprove toughnessPre-extraction tea treatmentGeraniolAdditive ingredient
The present invention relates to a black tea processing technology, and especially relates to a processing method of a flower fragrance-type black tea cake. In order to meet the requirement of consumers on flower fragrance-type tea, the method successively comprises the following steps: fresh leaf picking, heating for withering, rolling, constant-temperature withering, fermentation, pressing for cake forming, and drying. The black tea processed by adopting the method disclosed by the invention has strong flower and fruit fragrance, and is rich ingredients such as beta-linalool, methyl salicylate, trans-geraniol and methyl palmitate.
Owner:贵州钾天下茶业有限公司
Fermentation method of micafungin sodium intermediate
InactiveCN108753878ALow costPromote biosynthesisMicroorganism based processesFermentationSide chainFatty acid
The invention discloses a brand-new fermentation method of FR901379. According to the method, methyl palmitate is added as a side chain precursor of FR901379 fatty acid in the fermentation process ofFR901379 to promote biosynthesis of FR901379. At the same time, soybean lecithin insoluble in a reaction solution is introduced into a fermentation broth. Besides serving as a fermentation precursor,methyl palmitate also serves as an oxygen carrier in the fermentation system together with soybean lecithin, so that resistance of gas-liquid oxygen transfer is reduced, the problem of insufficient dissolved oxygen in the fermentation process in the prior art is solved, the fermentation yield of FR901379 is increased, and the production cost is reduced.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com