Serum combination marker for evaluating gliclazide applicability of type 2 diabetes mellitus and detection kit thereof
A type 2 diabetes, applicability technology used in analytical chemistry and clinical medicine and medicine to achieve good repeatability, improved data quality, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Serum sample collection
[0033] Before the collection of serum samples, all volunteers included in the study signed the informed consent.
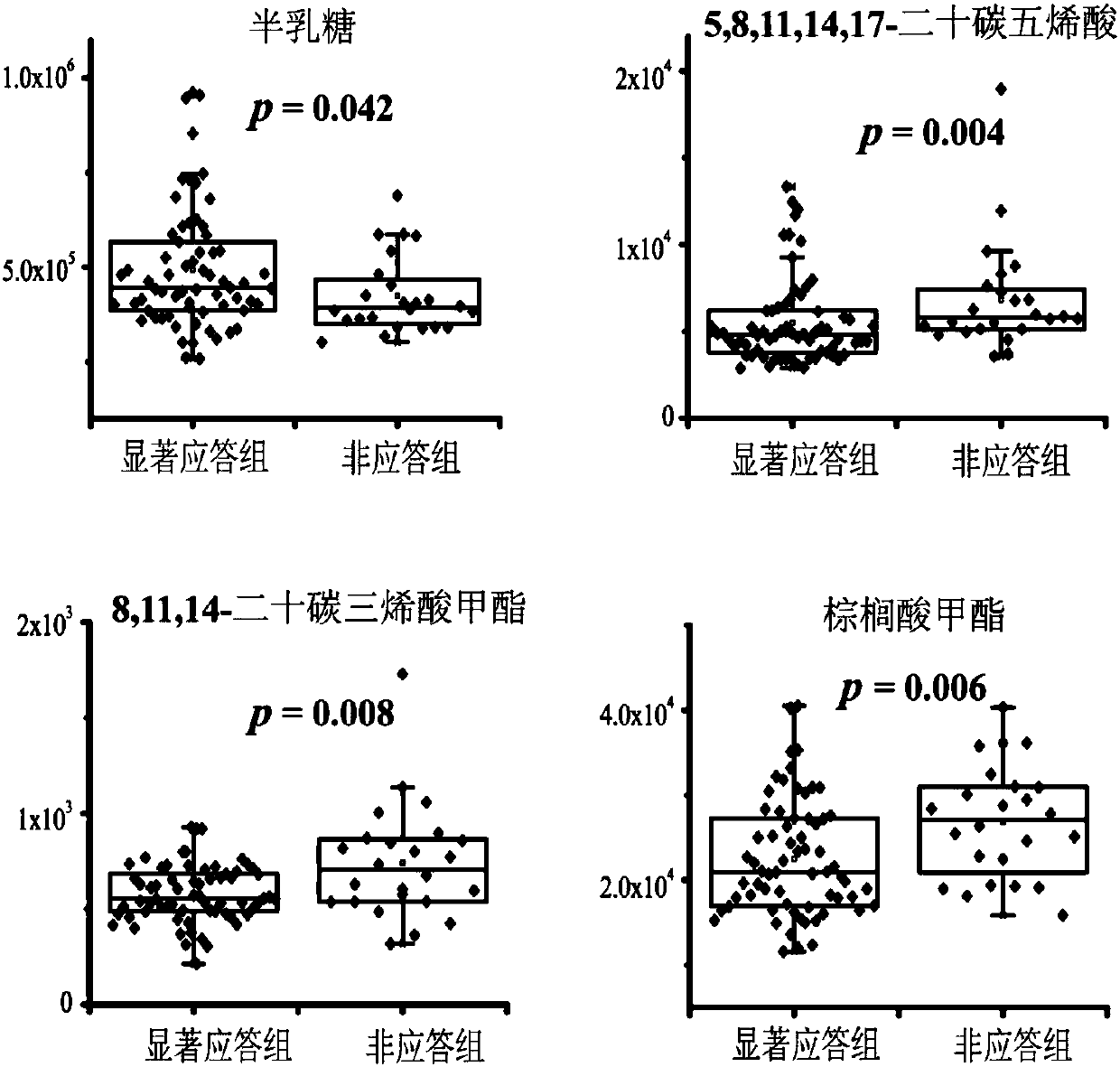
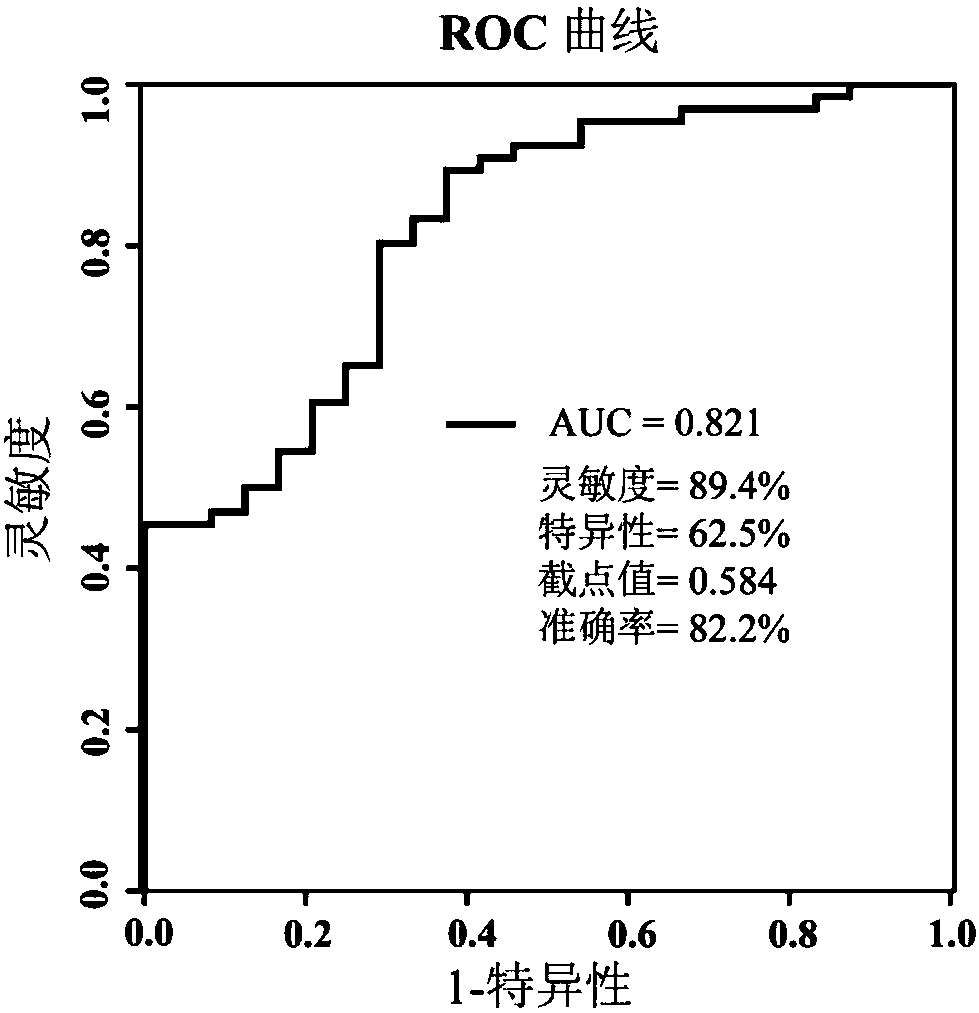
[0034] Including 100 patients with newly diagnosed type 2 diabetes, the diagnosis of type 2 diabetes refers to the criteria of the World Health Organization [Reference 10: Alberti, K.; Zimmet, P.Z.; Consultation, W.H.O. 1998, 15(7), 539-553]. Patients with type 1 diabetes, mitochondrial diabetes, or those taking antidiabetic drugs were excluded, as were patients with pregnancy, cancer, heart failure, or renal failure. After a 2-week lead-in period (diet and exercise only), 100 patients started treatment with gliclazide sustained-release tablets (baseline), and were followed up and evaluated clinically at weeks 2, 4, 8, 12, and 16. During this period, 10 patients were excluded because they did not complete the full course of treatment, and a total of 90 patients eventually completed 16 weeks of gliclazide treatment. Baseline se...
Embodiment 2
[0049] 1. Serum sample collection
[0050] Before the collection of serum samples, all volunteers included in the study signed the informed consent.
[0051]Including 26 patients with newly diagnosed type 2 diabetes, the diagnosis of type 2 diabetes refers to the criteria of the World Health Organization [Reference 10: Alberti, K.; Zimmet, P.Z.; Consultation, W.H.O. 1998, 15(7), 539-553]. Patients with type 1 diabetes, mitochondrial diabetes, or those taking antidiabetic drugs were excluded, as were patients with pregnancy, cancer, heart failure, or renal failure. After a 2-week lead-in period (diet and exercise only), 26 patients started treatment with gliclazide sustained-release tablets (baseline), and were followed up and evaluated clinically at weeks 2, 4, 8, 12, and 16. Baseline serum samples were collected from patients and stored in a -80°C refrigerator for future use.
[0052] 2. Analysis method
[0053] With embodiment 1.
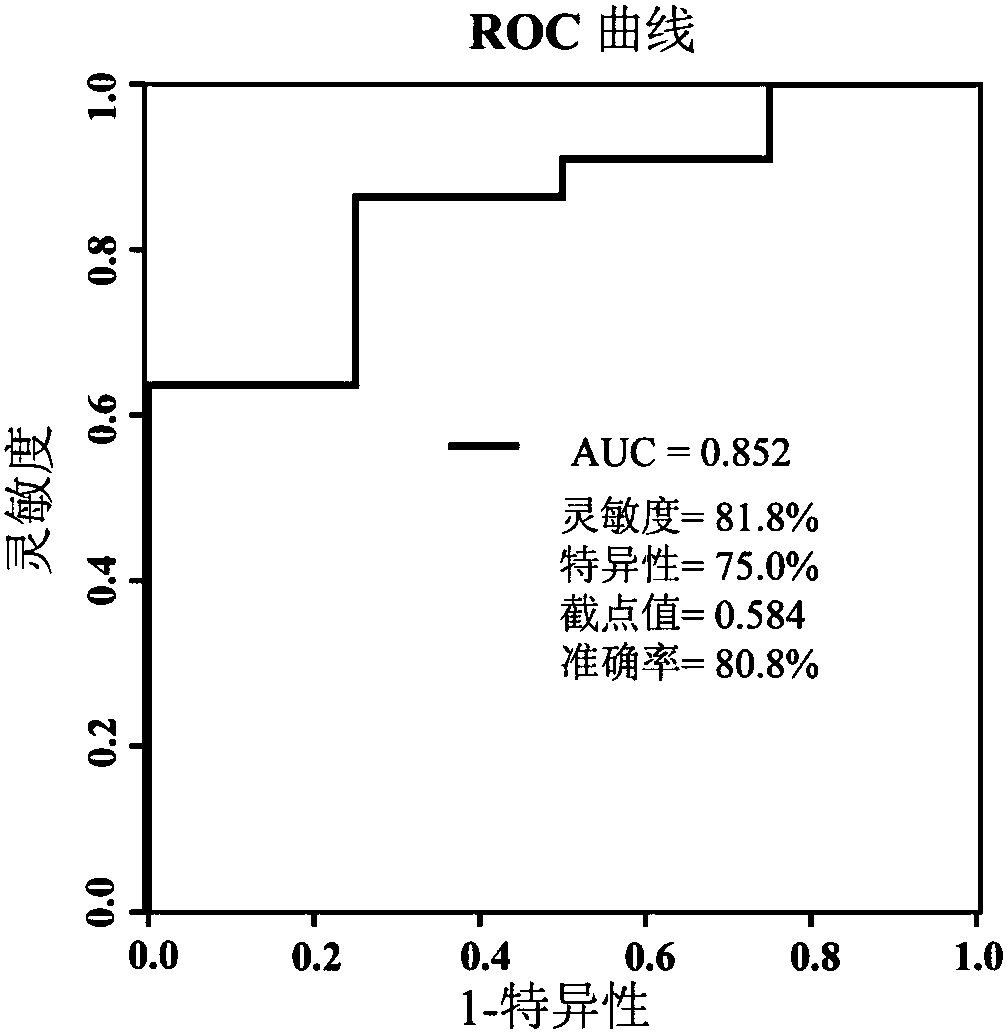
[0054] 3. Serum test results and auxili...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com