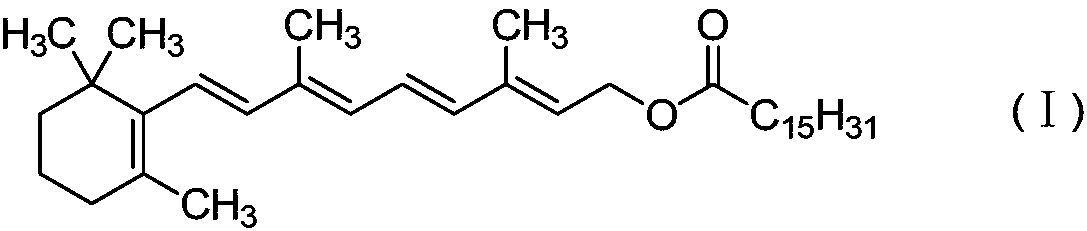
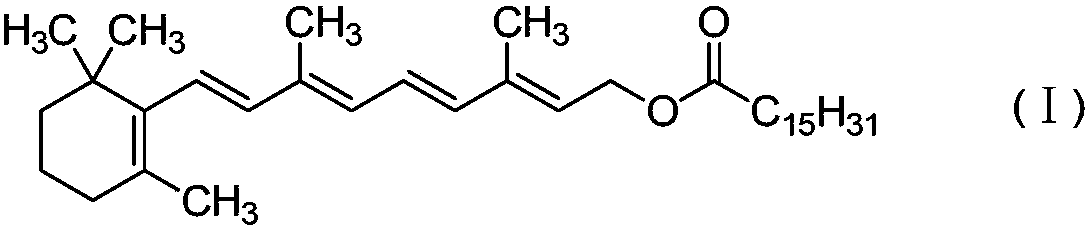
Preparation method of vitamin A palmitate
A technology of palmitate and vitamin, applied in the field of medicine, can solve the problems of low production capacity, difficult separation, no cost advantage, etc., and achieve the effects of less impurities, good appearance and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Add 328.50 g of vitamin A acetate and 1.65 g of sodium methoxide to 3.3 L of methanol under nitrogen protection, and react at 20°C for about 3 hours; after the reaction is complete, concentrate to dryness under reduced pressure at 40°C to obtain a yellow oily substance as vitamin A alcohol. The vitamin A alcohol obtained was mixed with 280.5 g of methyl palmitate, and the mixture was heated to 60° C., then the pressure was reduced to about 100 Pa, and the reaction was carried out for about 3 hours. Nitrogen gas was introduced to terminate the reaction, and 2 L of n-hexane and 16.5 g of activated carbon were added. After decolorization for 30 minutes, the mixture was filtered through a pad of silica gel, and the filtrate was concentrated to dryness under reduced pressure to obtain 488.2 g of light yellow oil, with a yield of 93%. The obtained oil was analyzed according to the method of USP28, and the result showed that the purity of vitamin A palmitate was 1.73 million IU...
Embodiment 2
[0020] Under the protection of nitrogen, add 328.50 g of vitamin A acetate and 3.28 g of sodium ethoxide to 3.3 L of ethanol, and react at 60°C for about 3 hours; after the reaction is completed, concentrate to dryness under reduced pressure at 45°C to obtain a yellow oily substance as vitamin A alcohol. The vitamin A alcohol obtained and 280.5 g of methyl palmitate were dissolved in 1 L of n-heptane, and the mixed solution was heated to 40° C., then the pressure was reduced to about 100 Kpa, and the reaction was carried out for about 3 hours. The reaction was terminated by passing nitrogen gas to obtain a reddish-brown oil; 2 L of n-hexane and 16.5 g of activated carbon were added, decolorized for 30 minutes, and then filtered through a pad of silica gel, and the filtrate was concentrated to dryness under reduced pressure to obtain 467.1 g of a light yellow oil with a yield of 89%. The obtained oil was analyzed according to the method of USP28, and the result showed that the p...
Embodiment 3
[0022] Add 164.3 g of vitamin A acetate and 3.28 g of potassium tert-butoxide to 1.6 L of methanol under nitrogen protection, and react at 50°C for about 3 hours; after the reaction is complete, concentrate to dryness under reduced pressure at 40°C to obtain a yellow oily substance as vitamin A alcohol. The vitamin A alcohol obtained and 140.3 g of methyl palmitate were dissolved in 1 L of toluene, and the mixed solution was heated to 40° C., then the pressure was reduced to about 10 Kpa, and the reaction was carried out for about 3 hours. The reaction was terminated by passing nitrogen gas to obtain a reddish-brown oil; 1 L of n-hexane and 8.2 g of activated carbon were added, decolorized for 30 minutes, and then filtered through a pad of silica gel. The filtrate was concentrated to dryness under reduced pressure to obtain 238.8 g of a light yellow oil, with a yield of 91%. The obtained oil was analyzed according to the method of USP28, and the result showed that the purity of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com