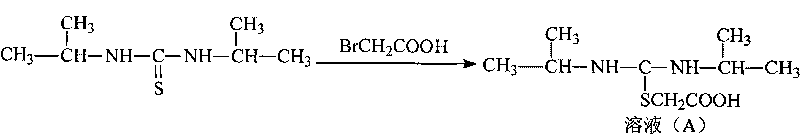Novel route for cefathiamidine compounds
A cefathiamidine and compound technology, which is applied in the field of synthesis of cephalosporins and cefathiamidine compounds, can solve the problems of deepening color, low content of crude products, instability under heat and the like, achieves improved purity and yield, and simple synthesis process , the effect of easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1. Synthesis of N, N'-Diisopropylamidine Thioacetic Acid
[0031] Add 320 grams of N,N'-diisopropylthiourea and 200 grams of sodium bicarbonate to a mixed solvent of 2 liters of acetone and 500 ml of water, stir for 10 min, then add 278 grams of 2-bromoacetic acid in 500 ml of Acetone solution was stirred and reacted at room temperature for 2 hours, then the pH of the reaction was adjusted to 3 with 2 mol / L hydrochloric acid, then cooled to 0° C., stirred to precipitate a solid, filtered, and dried to obtain 410 g of the product, with a yield of 94%.
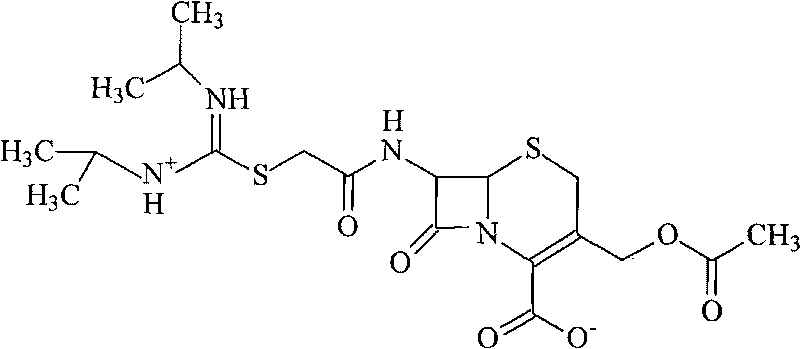
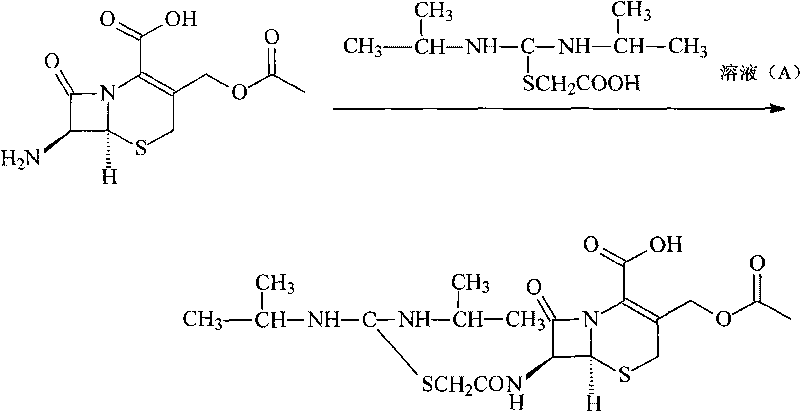
[0032] 2, the synthesis of cefathiamidine
[0033] Dissolve 510 grams of triphenylphosphine in 1 liter of 1,2-dichloroethane, add dropwise to 1 liter of 2-dichloroethane containing 550 grams of triphosgene, and keep the reaction temperature at 5°C, after the dropwise addition, react at this temperature for 2 hours, then add the mixture dropwise to 200 grams of N, N'-diisopropylamidinothioacetic acid in 800ml of 1,2-dichl...
Embodiment 2
[0038] 1. Synthesis of N, N'-Diisopropylamidine Thioacetic Acid
[0039] Add 640 grams of N, N'-diisopropylthiourea and 400 grams of sodium bicarbonate to a mixed solvent of 4 liters of acetone and 1000 ml of water, stir for 10 min, then add 1000 ml of 556 grams of 2-bromoacetic acid Acetone solution was stirred and reacted at room temperature for 2 hours, then the pH of the reaction was adjusted to 3.5 with 5 mol / L hydrochloric acid, then cooled to 0° C., stirred to precipitate a solid, filtered, and dried to obtain 813.9 g of the product, with a yield of 93.3%.
[0040] 2, the synthesis of cefathiamidine
[0041] 1020 grams of triphenylphosphine were dissolved in 2 liters of 1,2-ethylene dichloride, and added dropwise to 2 liters of 2-ethylene dichloride containing 1,100 grams of triphosgene, and the reaction temperature was maintained at 4°C, after the dropwise addition, react at this temperature for 2 hours, then add the mixture dropwise to 400 grams of N, N'-diisopropyla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com