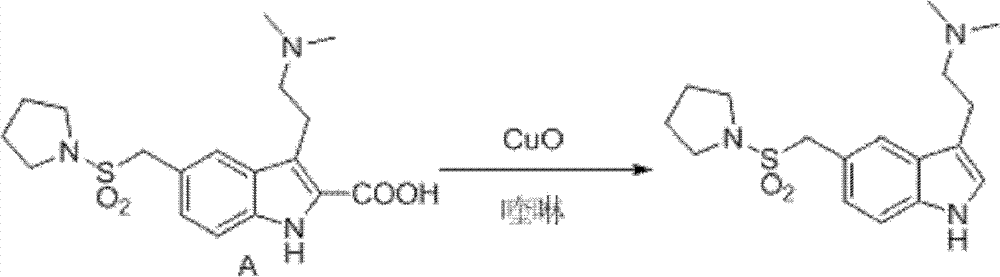
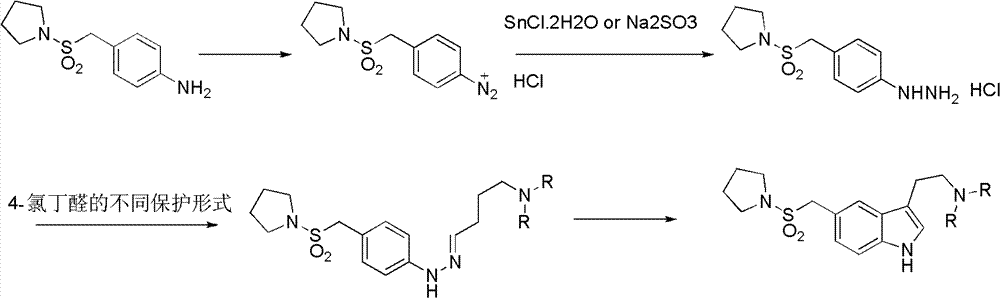
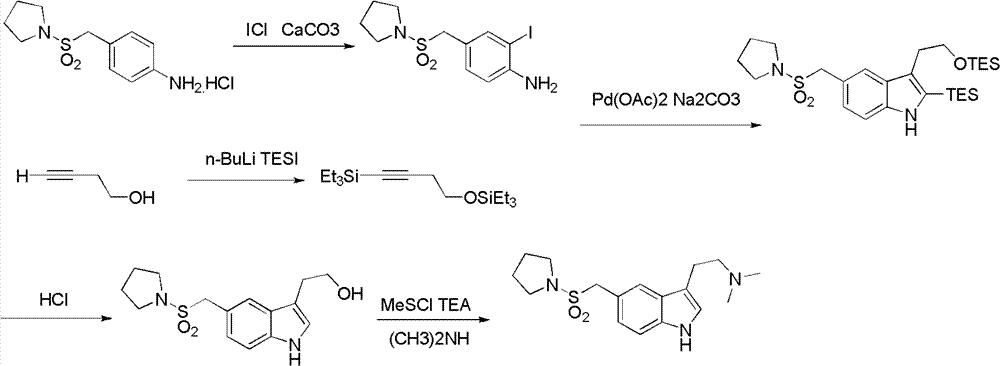
N-protection-3, 5-disubstituted indole derivative, its preparation method and application
A technology of indole derivatives and di-substitution, applied in the field of N-protected-3,5-disubstituted indole derivatives and their preparation and application, can solve the problems of expensive reagents, harsh reaction conditions, unfavorable large-scale production, etc. , to achieve the effects of not easy storage, shortened reaction steps, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] 2-[1-Benzyl-3-(2-dimethylaminoethyl)-5-indolyl]-2-sulfonylpyrrolidine]acetonitrile (III-1)
[0068] N 2 Under protection, add 2-(pyrrole-1-sulfonyl)acetonitrile (1.46g, 8.4mmo), toluene (7.2ml), ethylene glycol dimethyl ether (1.8ml) into a 25ml three-necked flask, and ice-bath to 0°C Add NaH (0.6g, 15mmol), stir at room temperature for 1h, ice-bath, add tetrakis(triphenylphosphinepalladium) (0.58g, 0.5mmol).
[0069] 2-[1-Benzyl-5-bromo-1H-indol-3-yl]-N,N-dimethylethylamine (2.4g, 6.72mmol) was dissolved in toluene and added dropwise to the above reaction solution , heated to 110°C, and reacted for 2h. Ice bath, add water and ethyl acetate to separate the liquid, dry the organic layer over anhydrous sodium sulfate, filter and concentrate, and separate through neutral alumina column (petroleum ether: ethyl acetate = 1:1, the same below) to obtain 2.73g of the product . Yield 90%.
[0070] 1 HNMR (400MHz, CDCl 3 ): 1.82(m, 4H), 2.03(s, 6H), 2.65(t, 2H), 2.93(t, 2H...
Embodiment 2
[0073] 2-[1-p-toluenesulfonyl-3-(2-dimethylaminoethyl)-5-indolyl]-2-sulfonylpyrrolidine]acetonitrile (III-2)
[0074] N 2 Under protection, add 2-(pyrrole-1-sulfonyl)acetonitrile (2g, 11.5mmo), toluene (24ml), ethylene glycol dimethyl ether (6ml) into a 100ml three-necked flask, ice-bath to about 0°C, add NaH (0.83g, 20.7mmol), stirred at room temperature for 1h, ice-bathed, added tetrakis(triphenylphosphine palladium) (0.8g, 0.69mmol).
[0075] 2-[1-p-toluenesulfonyl-5-bromo-1H-indol-3-yl]-N,N-dimethylethylamine (3.88g, 9.2mmol) was dissolved in toluene and added dropwise to the above reaction solution, heated to 80°C, reacted in an ice bath for 6 hours, added water and ethyl acetate to separate the layers, dried the organic layer over anhydrous sodium sulfate, concentrated by filtration, and separated by neutral alumina column to obtain 4.03 g of the product. Yield 85%.
[0076] 1 HNMR (400MHz, CDCl 3 ): 1.92(m, 4H), 2.26(s, 6H), 2.34(s, 3H), 2.55(t, 2H), 2.63(t, 2H), 2...
Embodiment 3
[0079] 2-[1-Trifluoroacetyl-3-(2-dimethylaminoethyl)-5-indolyl]-2-sulfonylpyrrolidine]acetonitrile (III-3)
[0080] N 2 For protection, add 2-(pyrrole-1-sulfonyl)acetonitrile (1.8g, 10.3mmo), nitrobenzene (18ml), ethylene glycol dimethyl ether (4.5ml) into a 50ml three-necked flask, and ice-bath to 0°C Add NaH (0.75g, 18.7mmol), stir at room temperature for 1h, ice-bath, add tetrakis(triphenylphosphine palladium) (0.7g, 0.6mmol).
[0081] 2-[1-Trifluoroacetyl-5-bromo-1H-indol-3-yl]-N,N-dimethylethylamine (3g, 8.2mmol) was dissolved in nitrobenzene and added dropwise to the above In the reaction solution, heated to 150°C, reacted for 0.5h, ice-bathed, added water and ethyl acetate to separate the liquid, the organic layer was dried over anhydrous sodium sulfate, concentrated by filtration, and separated by neutral alumina column to obtain 3.2g of the product. Yield 85%.
[0082] 1 HNMR (400MHz, CDCl 3 ): 1.92(m, 4H), 2.26(s, 6H), 2.51(t, 2H), 2.6(t, 2H), 2.83(m, 4H), 5.28(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com