Method for producing granular metal
a technology of granular metal and granular particles, which is applied in the direction of basic electric elements, rotary drum furnaces, electric devices, etc., can solve the problems of increasing the amount of by-product slag, affecting the cohesion property of slag, and imposing large limitations on the types of carbonaceous reductants, etc., to achieve long-term continuous operation, prolong the life of hearth refractories, and increase the rate of large metallic iron nu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0050] In this example, magnetite iron ore having a high gangue content, and more specifically, a high SiO.sub.2 content of 5.0% or more, was used as an iron oxide source. The chemical composition of the ore is shown in Table 1.
1TABLE 1 Chemical Composition of Material Iron Ore Having a High SiO.sub.2 Content (mass %) T.Fe Fe.sub.2O.sub.3 Fe.sub.3O.sub.4 SiO.sub.2 Al.sub.2O.sub.3 CaO MgO Other Total 67.21 1.15 91.77 5.4 0.28 0.81 0.45 0.14 100
[0051] The designed composition of high-SiO.sub.2 iron ore, a carbonaceous reductant, a binder (wheat flour), CaCO.sub.3 (basicity adjustor), and CaF.sub.2 (cohesion accelerator) in the material pellets was adjusted as shown in Table 2.
2TABLE 2 Designed Composition of the Material Pellets (mass %) Case A Case B (Comparative (Comparative Case C Case D Composition Example) Example (Example) (Example) High-SiO.sub.2 78.88 72.33 73.42 71.23 iron ore Coal 19.62 18.17 17.08 17.77 (carbonaceous reductant) Binder 1.5 1.5 1.5 1.5 CaCO.sub.3 0 8 7 8.5 Ca...
case b
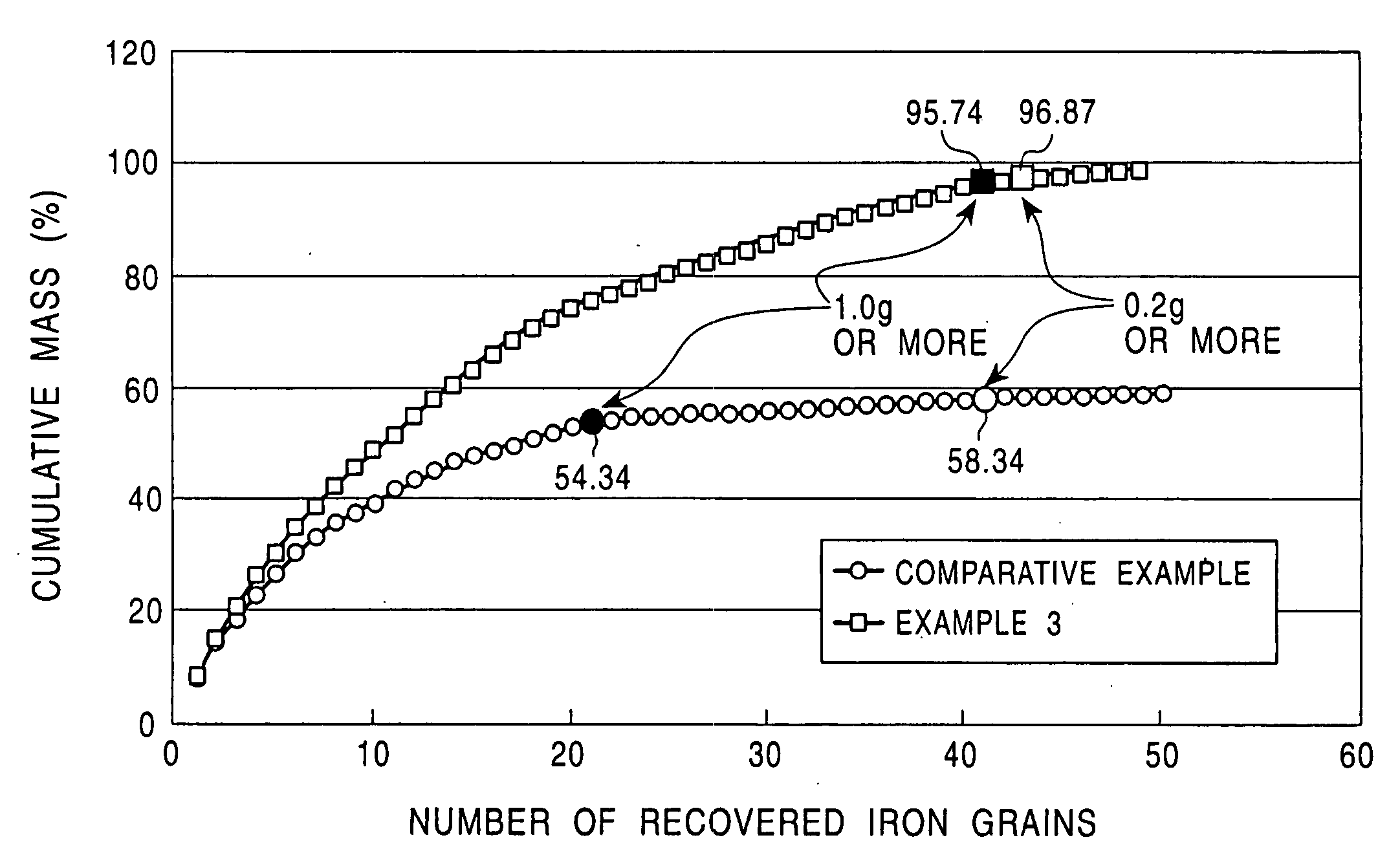
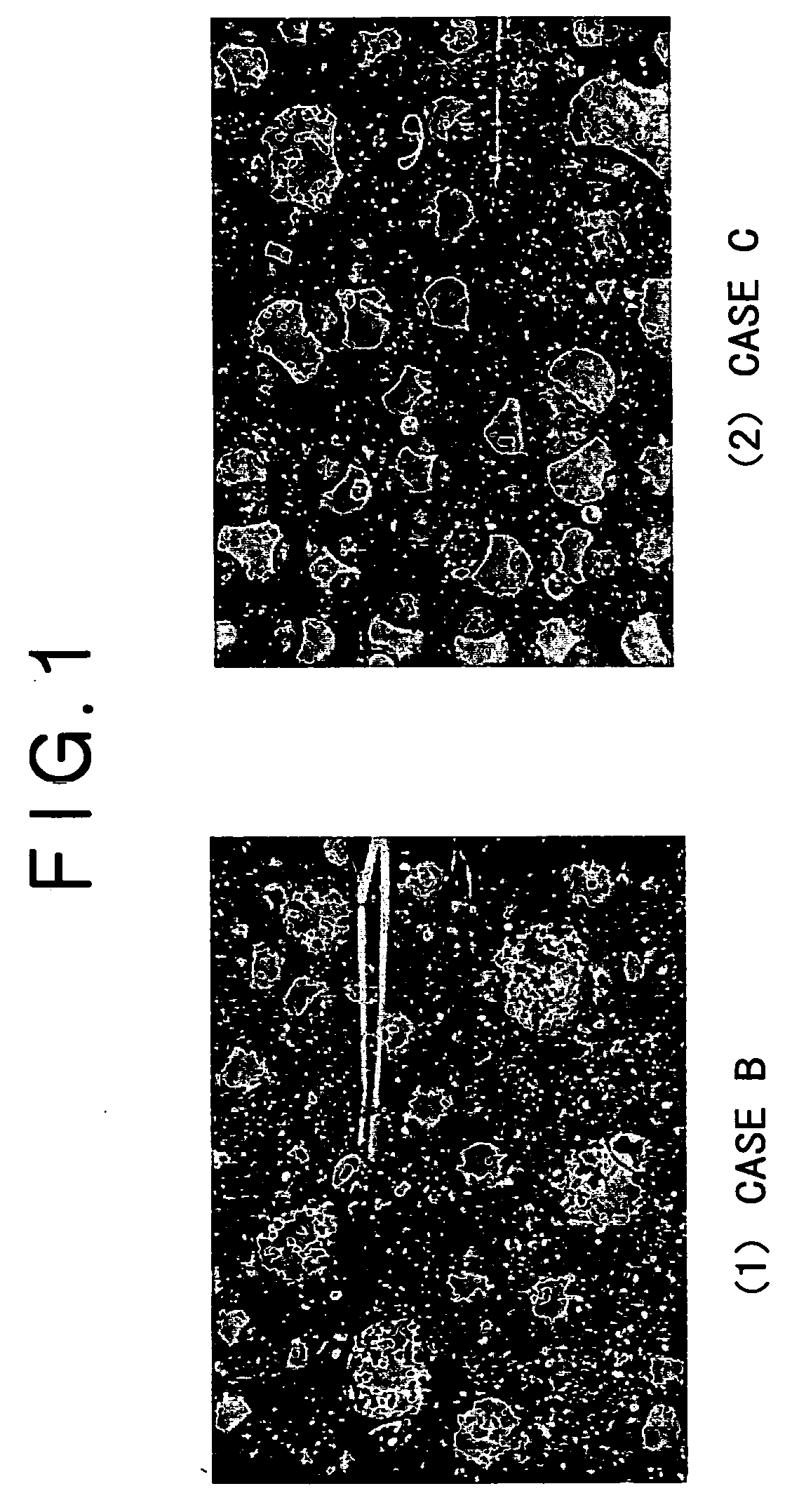
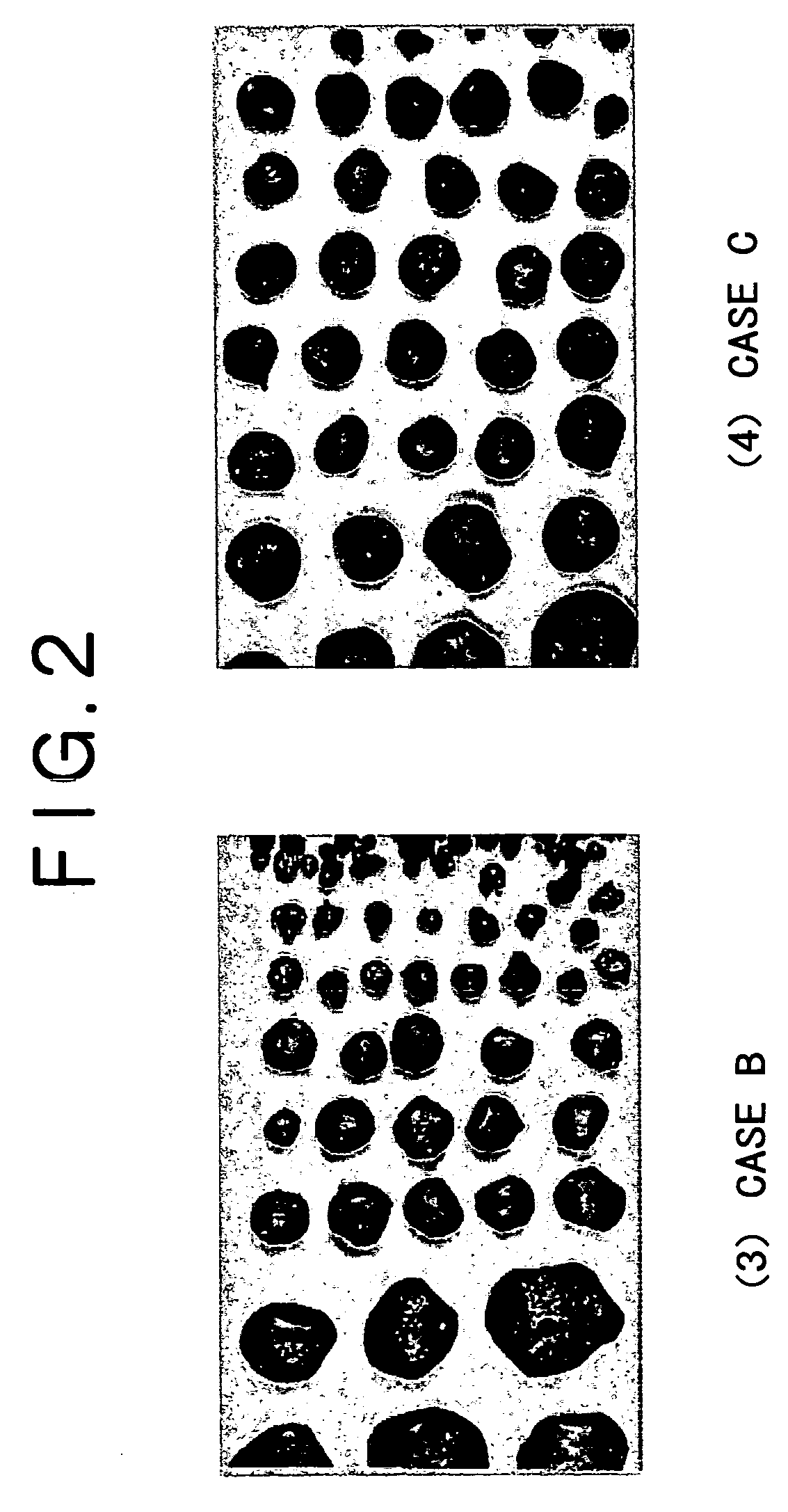
[0056] Case A was compared with Case B, in which the amount of the by-product slag was increased due to the addition of 8% of CaCO.sub.3, clearly demonstrated a tendency of an increase in the production rate of metallic iron nuggets having a small diameter. The recovery rates of the metallic iron nuggets of (1) 0.2 g or more and (2) 1.0 gram or more were clearly low. Particularly the recovery rate of the metallic iron nuggets having a mass of 1.0 g or more decreased by 12% or more. It should be noted that in Case B, large amounts of micro particles of metallic iron adhered onto the surface of the by-product slag, and those metallic iron nuggets that existed separately from the slag enclosed slag particles. Thus, the separation of the metallic iron nuggets from the slag was difficult.
[0057] On the other hand, when Case B was compared with Case C, although the amount of the by-product slag was increased in both Case C and Case B, Case C demonstrated that the cohesion of the molten me...
example 2
[0065] In this example, iron ore having a poor cohesion property, i.e., hematite ore, was used in the material to confirm the effect of adding approximately 1.0% of CaF.sub.2 as the cohesion accelerator. The experiments of reducing melt were conducted as in Example 1 above.
[0066] Table 6 shows the primary chemical composition of the hematite ore used in the experiment. FIGS. 3 and 4 include photographs showing the appearances of the state of the products immediately after the reducing melt (FIG. 3) on an aluminum board tray and the recovered metallic iron nuggets (FIG. 4) obtained in Case F which implements the present invention and uses CaF.sub.2 as the cohesion accelerator. FIGS. 3 and 4 also include photographs showing the appearances of the state of the products immediately after the reducing melt (FIG. 3) on an aluminum board tray and the recovered metallic iron nuggets (FIG. 4) obtained in Case E which is a comparative example that does not use CaF.sub.2.
6TABLE 6 Chemical Comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com