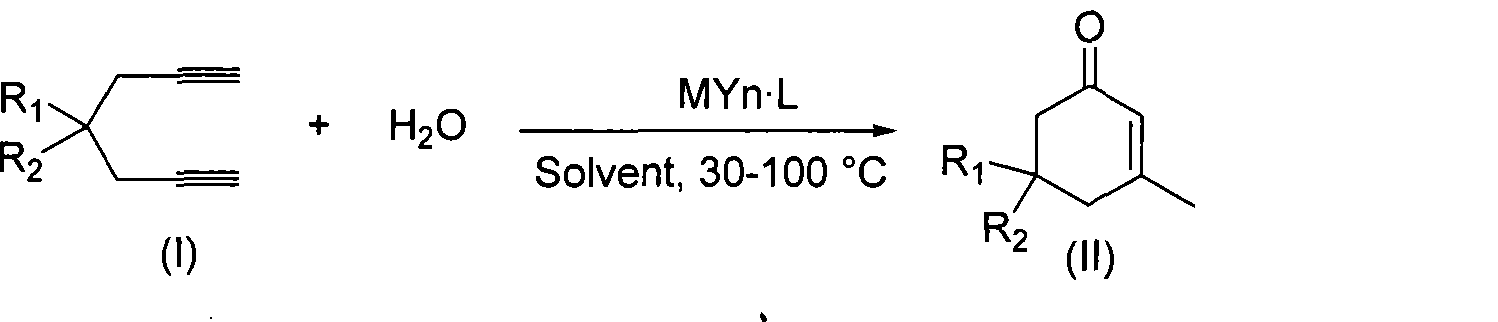
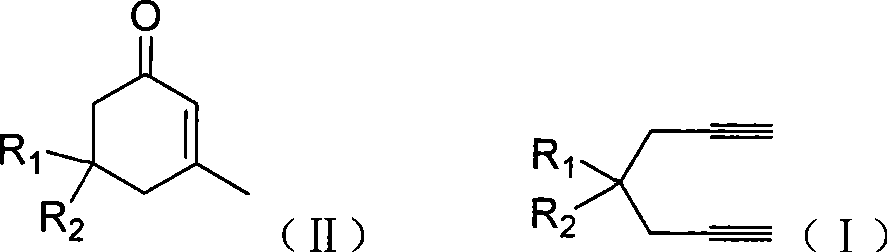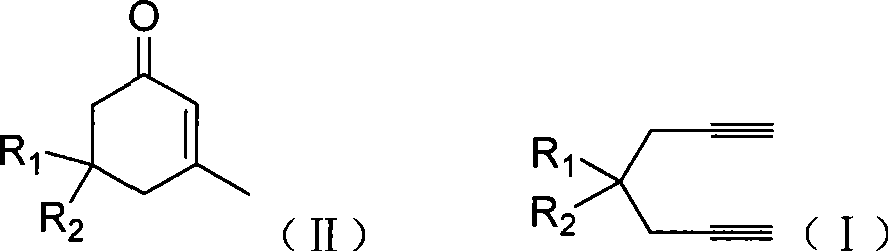Preparation of 2-pimelie kelone compound
A technology of cyclohexenones and cyclohexenones, which is applied in the field of preparation of 2-cyclohexenones, can solve problems such as the difficulty of introducing substituents at the 4-position, increasing reaction costs, and constraints, and achieves the goal of reaction The yield does not decrease, the prospect of wide application, and the effect of good reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of 3-methyl-5,5-dimethyl carboxylate-2-cyclohexenone
[0025] Dimethyl 2,2-dipropargyl malonate (104.1mg, 0.5mmol), gold complex (AuNO 3 ·PPh 3 , 12.5mg, 0.025mmol), methanesulfonic acid (20μL, 0.25mmol), water (10μL, 0.5mmol) in methanol (1.0mL) and the ionic liquid [Bmim]BF 4 (1.0 mL), and the mixture was heated at 70°C for 3 hours to react. After the reaction was completed, the methanol in the reaction solution was removed, and the organic matter (5×2 mL) in the ionic liquid was extracted with ether, and the remaining liquid after the extraction was the ionic liquid with the catalyst fixed, to be reused. After neutralizing methanesulfonic acid in the ether extract with saturated sodium bicarbonate, the organic layer was dried over anhydrous sodium sulfate. Diethyl ether was evaporated by filtration, and the obtained crude product was separated and purified by silica gel plate chromatography (petroleum ether: ethyl acetate = 5:1) to ob...
Embodiment 2
[0026] Embodiment 2: 3-methyl-5, the preparation of 5-dimethylcarboxylate group-2-cyclohexenone
[0027] Operation is with reference to embodiment 1, but nitrous group consumption is (AuNO 3 ·PPh 3 , 5mg, 0.01mmol), to obtain 81mg of the target compound (yield 72%).
Embodiment 3
[0028] Embodiment 3: the preparation of 3-methyl-5,5-dimethyl carboxylate group-2-cyclohexenone
[0029] Operation is with reference to embodiment 1, but nitrous group consumption is (AuNO 3 ·PPh 3 , 2.5mg, 0.005mol), to obtain 73mg of the target compound (yield 65%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com