Preparation method of N-formyl cefotaxime
A formyl head, cefotaxime acid technology, applied in the field of biological and pharmaceutical raw materials and intermediates, can solve the problems of unfavorable impurity research, etc., achieve the effect of simple and easy to control, improve product purity, and low product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: After successively adding 45g of acetic anhydride and 30g of anhydrous formic acid into a 1L dry and clean four-necked bottle, the temperature was raised to 25-35°C, and after stirring for 30 minutes, the temperature was lowered to 15°C, and 75g was added in 5 times After cefotaxime acid, the temperature rises to 35-40°C, when the reactants start to agglomerate and form a group, add 0.15g of zinc oxide and continue stirring for 30 minutes;
[0031] After the reaction, add 750mL of purified water, white crystals begin to precipitate, adjust the pH of the system to 3.0 with 15% ammonia solution, cool to 10-15°C and filter, then wash with 150mL of purified water, dry at 55°C, - Dry at 0.090Mpa for 5-10 hours until the water content is less than 2.5% to obtain the target product of white powder N-formyl cefotaxime, the mass yield of the target product is 80.2%, and the product purity (HPLC) is 97.9%.
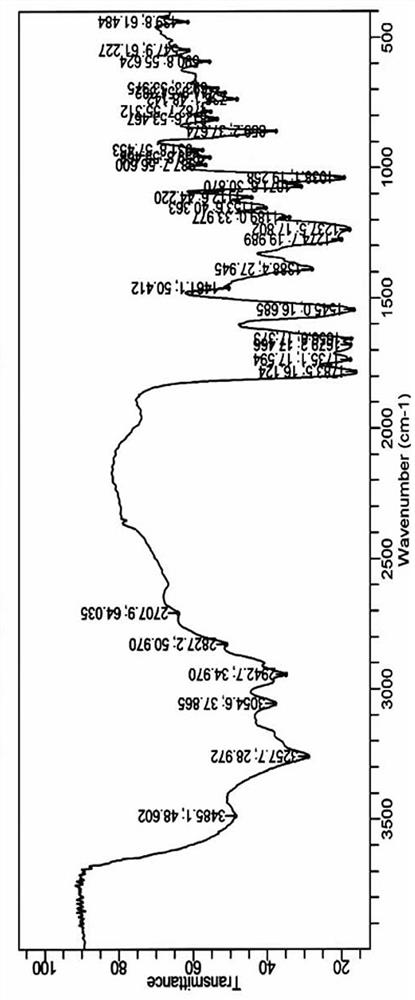
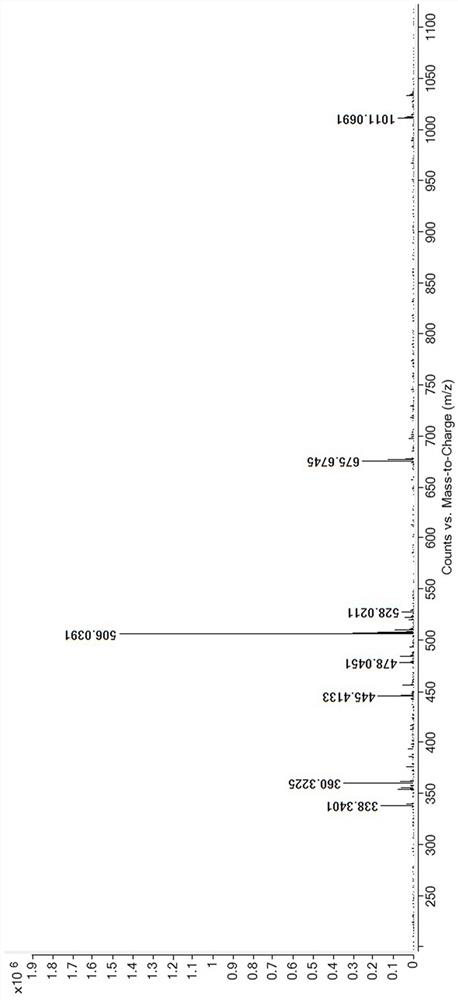
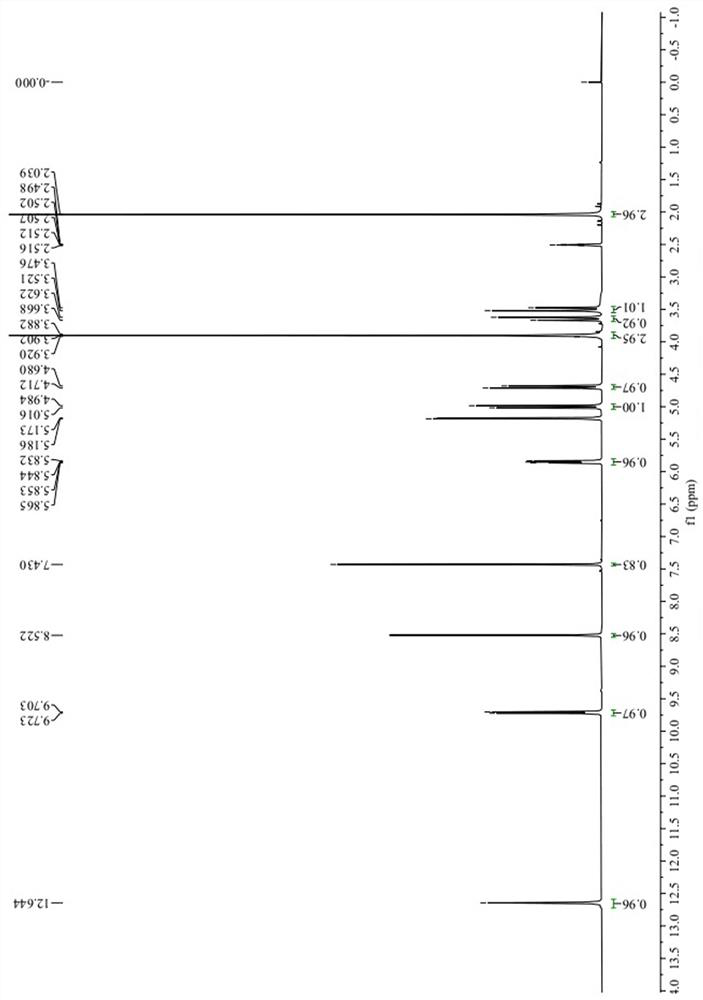
[0032] Structural analysis of the target product obtained abo...
Embodiment 2
[0038] Example 2: After successively adding 60 g of acetic anhydride and 30 g of anhydrous formic acid into a 2L dry and clean four-neck flask, the temperature was raised to 25-35°C, kept stirring for 30 minutes and then cooled to 15°C, and then 90g of cefotaxime was added in 3 times After the hydroxamic acid, the temperature rises to 35-40°C, and when the reactants start to agglomerate, add 0.10g of zinc oxide, and continue stirring for 30 minutes;
[0039] After the reaction, add 1100mL of purified water, white crystals begin to precipitate, use 20% ammonia solution to adjust the pH of the system to 2.5, cool to 10-15°C, filter, then wash with 200mL of purified water, dry at 55°C, - Dry at 0.090Mpa for 5-10 hours until the water content is less than 2.5% to obtain the target product of white powder N-formyl cefotaxime, the mass yield of the target product is 76%, and the product purity (HPLC) is 98.6%.
Embodiment 3
[0040] Example 3: After successively adding 96g of acetic anhydride and 30g of anhydrous formic acid into a 2L dry and clean four-necked flask, the temperature was raised to 25-35°C, and after stirring for 30 minutes, the temperature was lowered to 15°C, and 105g of cephalosporin was added in 3 times After thioxamic acid, the temperature rises to 35-40°C, when the reactants start to agglomerate, add 0.32g of manganese oxide, and continue to stir and react for 30 minutes;
[0041] After the reaction, add 1380mL of purified water, white crystals start to precipitate, use 18% ammonia solution to adjust the pH of the system to 3.5, cool to 10-15°C and filter, then wash with 300mL of purified water, dry at 55°C, - Dry at 0.090Mpa for 5-10 hours until the water content is less than 2.5% to obtain the target product of white powder N-formyl cefotaxime, the mass yield of the target product is 78%, and the product purity (HPLC) is 97.3%.
[0042] At present, the technical solution of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com