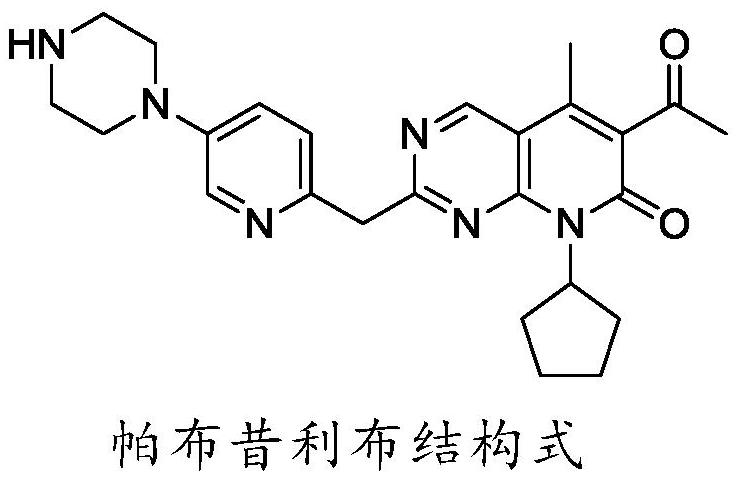
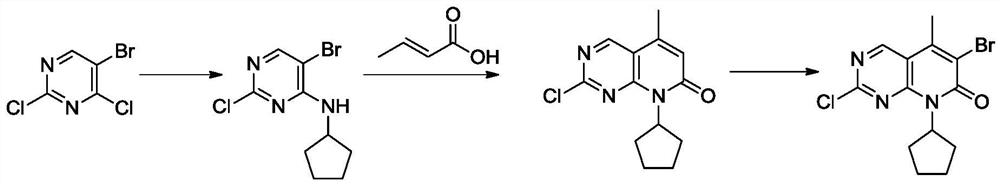
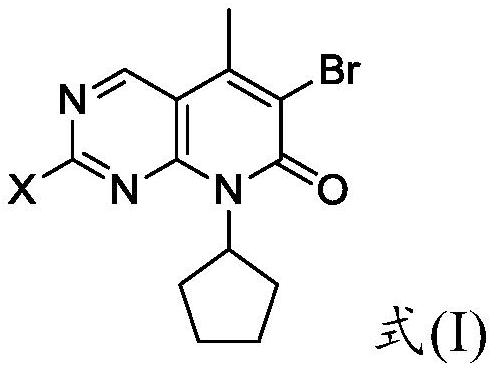
A kind of preparation method of palbociclib mother core structure compound
A technology of compound and mother nucleus, which is applied in the field of preparation of palbociclib mother nucleus structure compound, can solve the problems of difficult purification, many side reactions, and difficulty in obtaining, and achieve simple operation process, less side reactions and wide sources Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Preparation of 2-chloro-4-aminopyrimidine (intermediate 1)
[0061] Under the condition of mechanical stirring, add cytosine (57.05g, 0.5mol), phosphorus pentachloride (312.36g, 1.5mol) and 570mL of toluene into the reaction flask, stir well, heat up to 110°C for reflux reaction, TLC monitoring The reaction of the raw materials was completed; concentrated under reduced pressure to remove most of the solvent, lowered to room temperature, added 500 mL of water, extracted several times with dichloromethane, combined the organic phases, dried with anhydrous sodium sulfate to remove water, concentrated under reduced pressure to remove dichloromethane, and obtained Light yellow transparent liquid intermediate 1 (62.84g, yield 97%, purity 97.7%), directly used in the next step without purification; or further, add 250ml of n-hexane in an ice-water bath, stir and crystallize for 2h, suction filter, and a small amount of n-hexane Rinse and dry in vacuo to obtain ligh...
Embodiment 2
[0063] Example 2 Preparation of 5-bromo-2-chloro-4 aminopyrimidine (intermediate 2)
[0064] Add 500 mL of glacial acetic acid to the light yellow transparent liquid of Intermediate 1 and stir to dissolve, add KBr (6.2 g, 10%), slowly add bromine (81.4 g, 0.51 mol) in 80 mL of glacial acetic acid solution dropwise at room temperature, and the addition is complete , the reaction system turns vermilion, slowly warming up to 55°C for 1h, the reaction solution turns from vermilion to white, and a solid precipitates; continue to react for 1h, cool down to room temperature, add 10ml of saturated aqueous sodium bisulfite solution, and continue stirring for 0.5h , filtered with suction, rinsed with purified water, and dried under vacuum at 45°C to obtain white solid powder intermediate 2 (97.06g, yield 96%, purity 98.73%); 1 H NMR (400MHz, CDCl 3 )δ: 8.71(s,1H), 5.77(s,2H).
[0065] When changing the amount of intermediate 1 and brominating reagent so that the molar ratio is in t...
Embodiment 3
[0066] Example 3 Preparation of 5-bromo-2-chloro-4-N-cyclopentylpyrimidine (intermediate 3)
[0067] Intermediate 2 (83.4g, 0.4mol), triethylamine (40.5g, 0.4mol) and methanol 300ml were added to the reaction flask and stirred to dissolve, and chloropentane (50.2g, 0.48mol) was added dropwise at room temperature, and After the addition is completed, the temperature is controlled and stirred for reaction 2, and TLC is monitored until the reaction is completed. Add 350ml of ice water, stir and crystallize at a temperature of 0-10°C for 1 hour, filter with suction, rinse with ice water, drain, and vacuum dry at 45°C to obtain a white solid powder Intermediate 3 (102.57g, yield 92.74%, purity 99.18%), 1 H NMR (400MHz, CDC1 3 )δ: 8.10 (s, 1H), 5.47 (d, J = 4.5Hz, 1H), 4.42 (dd, J = 14.0, 7.0Hz, 1H), 1.44 ~ 2.17 (m, 8H).
[0068] When changing the amount of intermediate 2 and halocyclopentane so that the molar ratio is in the range of 1:1.18-1:1.25, the yield of intermediate 3 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com