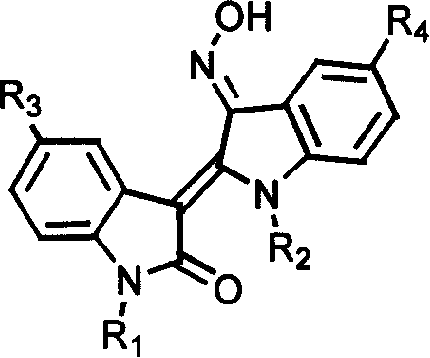
Specific indole compound and its preparation and use in treating and preventing cancers
A technology of compounds, indoles, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
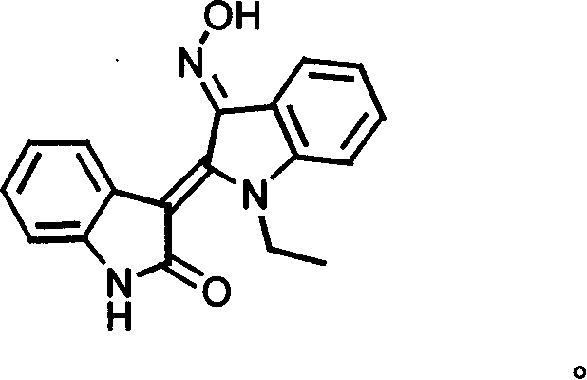
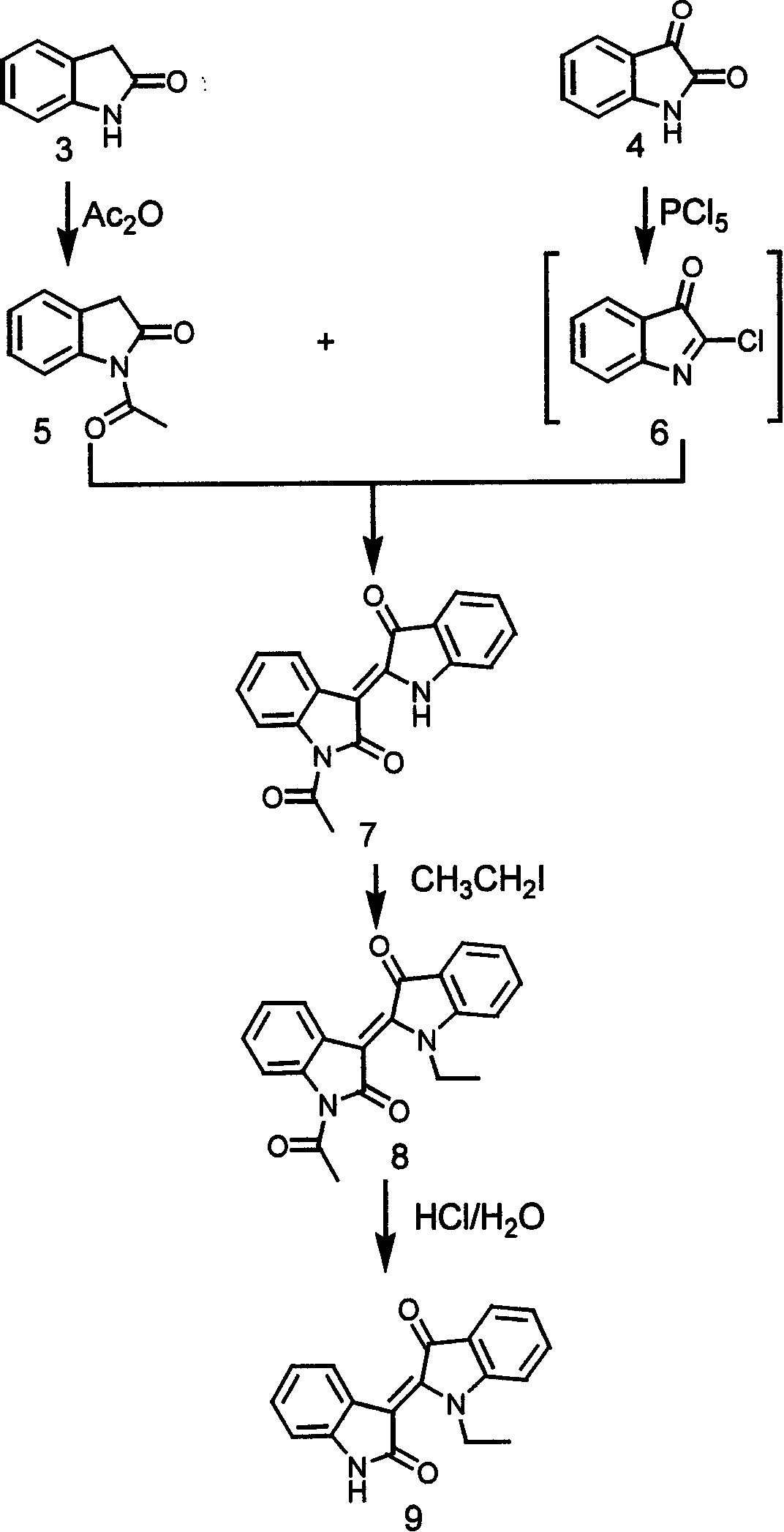
[0081] Experimental Example 1: Chemical Synthesis and Structure Confirmation of JN-2518
[0082] (1) Materials and methods
[0083] 1. Reagents: 2-keto indole (Oxindole), indoline diketone (Isatin), iodoethane, phosphorus pentachloride, hydroxylamine hydrochloride, acetic anhydride and toluene and other reagents were purchased from Sigma-Aldrich Chemical Reagent Company in the United States .
[0084] 2. Method: 2-ketoindole (3, Oxindole) was refluxed in acetic anhydride to obtain nitrogen acetylated product 5. Indoline dione (4, Isatin) and phosphorus pentachloride reaction in benzene solution obtained chlorinated intermediate 6 and compound 5 in anhydrous toluene immediately backflow 2 hours, after obtaining solid filtration, use toluene and ethanol successively Wash statically, recrystallize in dimethylamide to obtain compound 7, and then react with ethyl iodide under alkaline conditions to obtain nitrogen alkylation reaction product 8, and nitrogen ethylated product 8 is...
experiment example 2
[0087] Experimental example 2: Inhibitory effect of JN-2518 on human cancer cells
[0088] The following are some examples of JN-2518 and other priority compounds in the treatment of tumors. Here again, we reiterate that although we only use the treatment of tumors as an example, it does not mean that these compounds are only used to treat tumors.
[0089] (1) Materials and methods
[0090] Reagent: JN-2518 and its series of compounds, synthesized by our laboratory, confirmed the structure by mass spectrometry, NMR, infrared, and purified by high pressure liquid chromatography (HPLC). The purity was >98.0%. JN-2518 is a dark red crystalline powder with a molecular weight of 305. During the experiment, a solution was prepared with DMSO and stored at -20°C. Retinoic acid, daunorubicin, and paclitaxel were purchased from Sigma Chemical Reagent Company in the United States. NS389, a novel cyclooxygenase II inhibitor, was purchased from Biomol. Research Lab, USA. Unless other...
experiment example 3
[0107] Experimental example 3: The effect of JN-2518 on prostate cancer ascites metastasis cancer cells:
[0108] (1) Materials and methods
[0109] Reagent: JN-2518 see Experimental Example 1. Daunorubicin, paclitaxel, arsenic trioxide (As 2 o 3 ), Triochstatin A (TSA) was purchased from Sigma Chemical Reagent Company, USA. Unless otherwise specified, other chemical reagents used in the experiment were purchased from Sigma Chemical Reagent Company in the United States.
[0110] Cell culture: the primary cultured human cancer cell B1011 transferred from the ascites of prostate cancer was collected from the patient's ascites by centrifugation, and stored at 37°C, 5% CO 2 In an incubator, culture in RPMI1640 medium containing 10% fetal bovine serum and penicillin-streptomycin. After one week in culture, the cells grew rapidly and steadily. B1011 cancer cells in logarithmic growth phase were taken to study the anticancer activity of JN-2518 by MTT method.
[0111] MTT: The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com