Method for preparing hexafluorobutadiene from iodine and chlorine
A technology of hexafluorobutadiene and hexafluorobutane, which is applied in the field of hexafluorobutadiene preparation, can solve the problems of long reaction time, many side reactions, low catalytic efficiency, etc., and achieves a long reaction time and many side reactions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
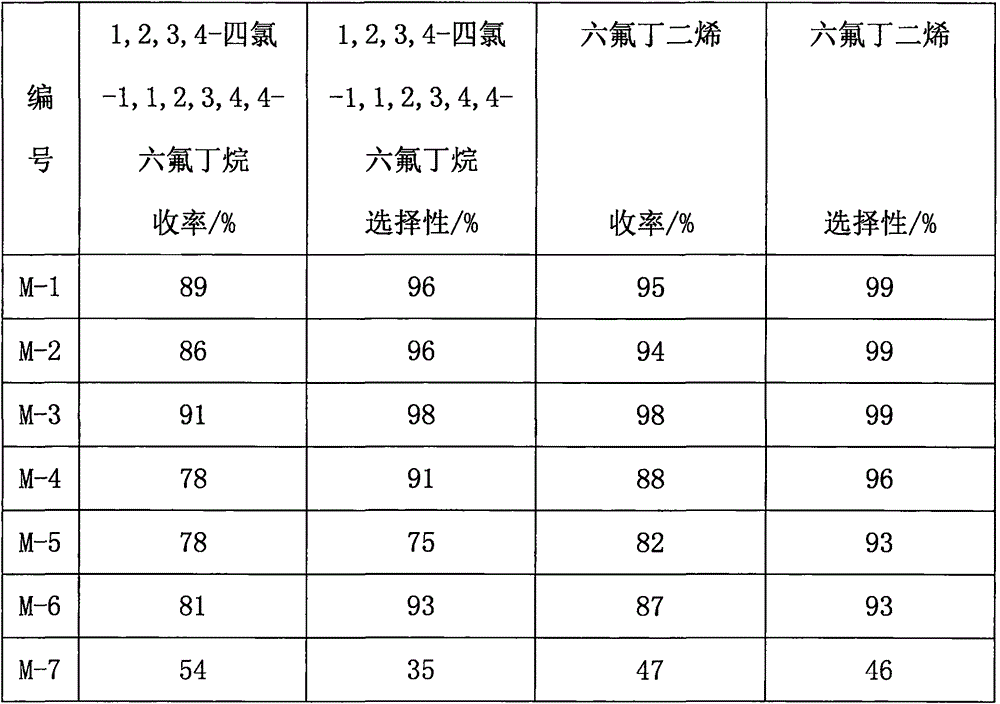
Examples
Embodiment 1
[0032] (1) Preparation of metal complex ionic liquid solvent:
[0033] Add 100Kg ethanol, 0.05Kg vitamin B12 (Vitamin B12), 0.2Kg 1-ethyl ethyl ether-3-methylimidazolium tetrafluoroborate in 500L reactor, 1Kg boron trifluoride ether solution, add Mix in the reaction kettle at room temperature for 20 hours to prepare the metal complex ionic liquid solvent;
[0034] (2) Preparation of iodine monochloride:
[0035] Add 100Kg iodine in a 500L reactor, the reaction temperature is 58°C, iodine is sublimated, chlorine gas is introduced, the molar ratio of chlorine gas and iodine is 1.2:1, the reaction end point is that the solid is completely transformed into a liquid, and the obtained liquid is distilled to obtain monochloride iodine products.
[0036] (3) Preparation of 1,2-dichloro-2-iodo-1,1,2-trifluoroethane:
[0037] In the 500L reactor, add 100Kg iodine monochloride, iodine monochloride, chlorotrifluoroethylene is put into reactor with mol ratio 1.2: 1, adds the metal compl...
Embodiment 2
[0043] (1) Preparation of metal complex ionic liquid solvent:
[0044] Add 100Kg1,4-dioxane, 0.01Kg vitamin B12 (Vitamin B12), 0.05Kg 1-ethyl ethyl ether-3-methylimidazole tetrafluoroborate, 0.5Kg tri The boron fluoride ether solution was added to the reaction kettle and mixed at room temperature for 10 hours to obtain the metal complex ionic liquid solvent;
[0045] (2) Preparation of iodine monochloride:
[0046] Add 100Kg iodine in a 500L reactor, the reaction temperature is 55°C, iodine is sublimated, chlorine gas is introduced, the molar ratio of chlorine gas and iodine is 1.1:1, the reaction end point is that the solid is completely transformed into a liquid, and the obtained liquid is distilled to obtain monochloride iodine products.
[0047] (3) Preparation of 1,2-dichloro-2-iodo-1,1,2-trifluoroethane:
[0048] In the 500L reactor, add 100Kg iodine monochloride, iodine monochloride, chlorotrifluoroethylene is put into reactor with mol ratio 1.1: 1, adds the metal co...
Embodiment 3
[0054] (1) Preparation of metal complex ionic liquid solvent:
[0055] Add 60Kg tetrahydrofuran, 40Kg propanol, 0.1Kg vitamin B12 (VitaminB12), 0.5Kg 1-ethyl ethyl ether-3-methylimidazolium tetrafluoroborate, 2Kg boron trifluoride ether in 500L reactor solution, added to the reactor at room temperature and mixed for 40 hours to obtain the metal complex ionic liquid solvent;
[0056] (2) Preparation of iodine monochloride:
[0057] Add 100Kg iodine in a 500L reactor, the reaction temperature is 70°C, iodine is sublimated, chlorine gas is introduced, the molar ratio of chlorine gas and iodine is 1.5:1, the end point of the reaction is that the solid is completely transformed into a liquid, and the obtained liquid is distilled to obtain monochloride iodine products.
[0058] (3) Preparation of 1,2-dichloro-2-iodo-1,1,2-trifluoroethane:
[0059] In the 500L reactor, add 100Kg iodine monochloride, iodine monochloride, chlorotrifluoroethylene is put into reactor with mol ratio 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com