Method for separating and measuring bilastine and technical impurities in preparation of bilastine
A technology of process impurities and preparations, applied in the field of analytical chemistry, can solve the problems of time-consuming, etc., and achieve the effect of quality control and shortened separation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
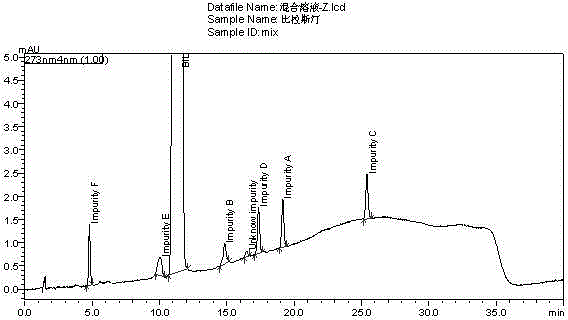
[0048] Preparation of the mixed solution: Weigh about 40mg of the test product, put it in a 25ml measuring bottle, precisely pipette 2.5ml of the impurity A-F reference substance solution, put it in the same measuring bottle as above, add diluent to dissolve and dilute to the mark, shake well, Instantly.
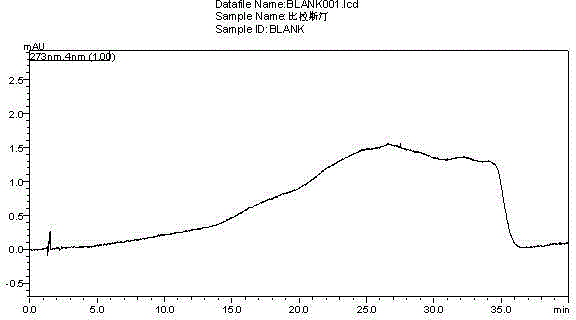
[0049] The diluent and the mixed solution were respectively injected according to the above-mentioned chromatographic conditions, and the chromatogram was recorded. The measurement results are shown in Table 2. see results figure 1 , figure 2 .
[0050] Table 2 test results
[0051]
[0052] Conclusion: The blank diluent does not interfere with the sample determination; the separation between the main peak and adjacent impurity peaks is greater than 1.5; the separation between the known impurity peaks is greater than 1.5; the above tests prove that the main peak and impurity peaks are well separated and specific.
[0053] comparative example
[0054] In this comparat...
Embodiment 2
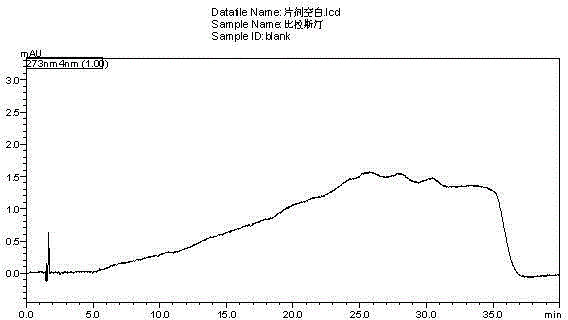
[0059] Get need testing solution, sample injection by chromatographic conditions in embodiment 1, record chromatogram, and carry out blank adjuvant test in the same way, the results are shown in image 3 , Figure 4 .
[0060] Conclusion: blank excipients do not interfere with the determination of this product, indicating that the method of the present invention can be used for the quality detection of bilastine tablets.
[0061] Embodiment 3 Bilastine oxidative degradation product determination
[0062] Take 40mg of bilastine, weigh it accurately, put it in a 25ml measuring bottle, add 2.0ml of 3% hydrogen peroxide, bathe in water at 30°C for 1.5h, take it out, cool it, dissolve it with a diluent and dilute to the mark, shake it up, and measure it as a degradation product with solution.
Embodiment 3
[0063] Get the above-mentioned degradation product measurement solution, sample injection by the chromatographic conditions in Example 1, record the chromatogram, do blank test simultaneously, record the chromatogram, as Figure 5 As shown, the impurity content was calculated according to the area normalization method. The test results are shown in Table 3.
[0064] Table 3 Oxidative Degradation Test Determination Results
[0065]
[0066] Conclusion: Under the condition of oxidative degradation, impurity E was produced in 30℃ water bath for 1.5h, the level was 2.16%, and the original impurity had no change trend. The separation between the main peak and adjacent impurity peaks was greater than 1.5, and the purity factor of the main peak was 0.9999. This shows that the chromatographic conditions are good for the separation of oxidative degradation products.
[0067] Example 4 Basic research on detection limit and quantitative limit of bilastine and impurities A-F in chro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com