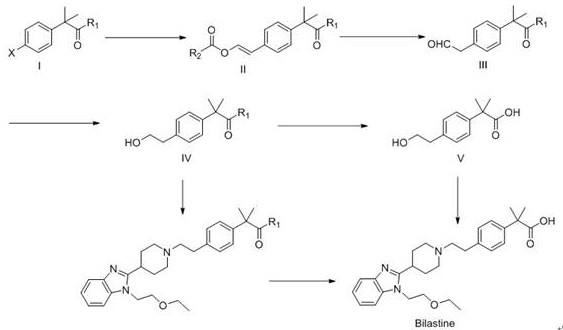
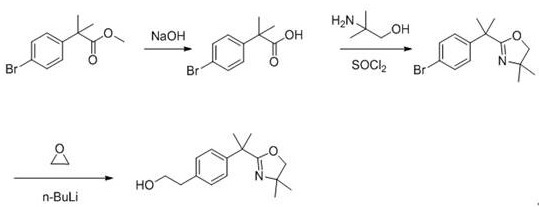
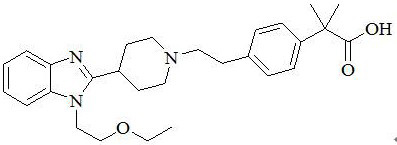
Preparation method of bilastine intermediate
A preparation process and compound technology, applied in the field of preparation of bilastine intermediate 2-phenyl)-2-methylpropionic acid and its derivatives, can solve the problems of easy volatility, difficult industrial production, low yield, etc. problems, to achieve the effect of mild reaction conditions, no side reactions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: Synthesis of 4-(1-(dimethylamino)-2-methyl-1-oxopropan-2-yl)styrene acetate
[0022] Dissolve 81.04g (0.3 mol) of 2-(4-bromophenyl)-N,N,2-trimethylpropanamide in 600ml of anhydrous tetrahydrofuran, cool down to -10°C, stir to dissolve, and replace with nitrogen for 3 times 1.01g (0.045 mol) of palladium acetate was added to the system in batches. After the addition, 68.87g (0.8 mol) of vinyl acetate was dissolved in 200ml of anhydrous tetrahydrofuran, and added dropwise to the reaction system. After the addition was completed, it rose to Continue to react at room temperature for 5 hours, TLC monitors that the raw material point basically disappears. After the reaction is completed, it is suction filtered, and the filtrate is washed with deionized water to separate the liquid. After drying, it is concentrated to obtain a light yellow oil with a yield of 92.51%, which is directly put into the next reaction.
Embodiment 2
[0023] Example 2: Synthesis of N, N, 2-trimethyl-2-(4-(2-oxoethyl)phenyl)propionamide
[0024] With 5.0 g of the compound in Example 1, use 1 mol / L sodium hydroxide solution as a solvent, stir at room temperature for 24 h, TLC monitors that the raw material point disappears, extract 3 times with 50 ml of dichloromethane, concentrate the organic phase, add 15 ml of petroleum ether and stir in an ice-water bath, Suction filtration gave 3.85 g of a waxy solid with a yield of 90.8%, and the product was stored at low temperature.
Embodiment 3
[0025] Example 3: Synthesis of 2-(4-(2-hydroxyethyl)phenyl)-N,N,2-trimethylpropionamide
[0026] Dissolve 12.0 g (0.05 mol) of the compound prepared according to Example 2 in ethanol, add 4.53 g (0.12 mol) of sodium borohydride in batches, stir at room temperature for 4 hours, slowly pour the reaction solution into 10% citric acid aqueous solution, add After completion, continue to stir for 30 minutes, spin off the ethanol in the system, then extract the reaction solution with dichloromethane, adjust the organic phase to neutrality with saturated aqueous sodium bicarbonate solution, then separate and dry, add ethyl acetate 4.8 g after the organic phase is concentrated ml and 15ml of petroleum ether were stirred and crystallized, and 10.6g of a light yellow solid was obtained by suction filtration, with a yield of 87.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com