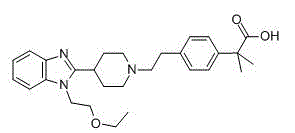
Bilastine compound
A bilastine and compound technology, applied in the field of bilastine compounds and their preparation, can solve the problems of no anticholinergic effect, a large number of bilastine impurities, difficult to pass through the blood-brain barrier and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] In a 500ml reaction flask equipped with stirring, thermometer and condenser, add 60 grams of bilastine and 240 milliliters of water, add 0.05 grams of dimethylformamide (DMF) to the above aqueous solution, stir for 40 minutes, filter, and the filtrate Cool to 8°C and set aside.
[0050] Cool 900ml of acetonitrile-methyl ethyl ketone = 7:5 mixture to 8°C, add the above standby solution while stirring, keep warm for 18 hours, crystals precipitate, filter, and dry to obtain 53.3 grams of white crystals. Purity 99.9% (HPLC normalization method), optical purity 99.96% ee (chiral HPLC).
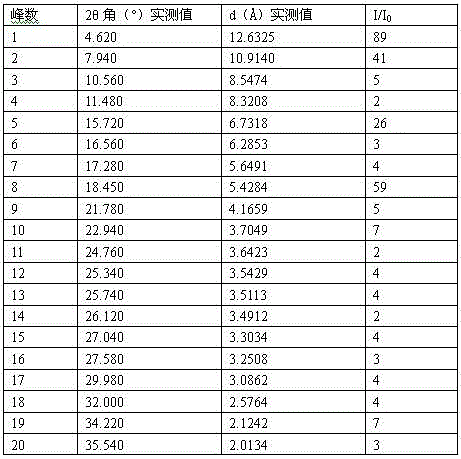
[0051] This crystal was subjected to X-ray diffraction measurement. Instrument model and measurement conditions: Rigaku D / max 2500 diffractometer; CuKa 40Kv 100mA; 2θ scanning range: 0-50 ° . The result is as follows:
[0052]
Embodiment 2
[0054] Capsules containing bilastine compound
[0055] Prescription: 20 grams of bilastine compound, 100 grams of mannitol, 140 grams of lactose, 50 grams of calcium carbonate, 5 grams of magnesium stearate, appropriate amount of 8% ethyl cellulose solution, 1000 enteric-coated capsules, made into 1000 capsules .
[0056] Process: Mix bilastine compound, mannitol, lactose, and calcium carbonate evenly; add an appropriate amount of binder 8% ethyl cellulose solution to make a soft material, granulate with a 20-mesh screen, and dry at 40°C±2°C. Whole grains, add externally mixed magnesium stearate, mix evenly, pack into capsules, and obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com