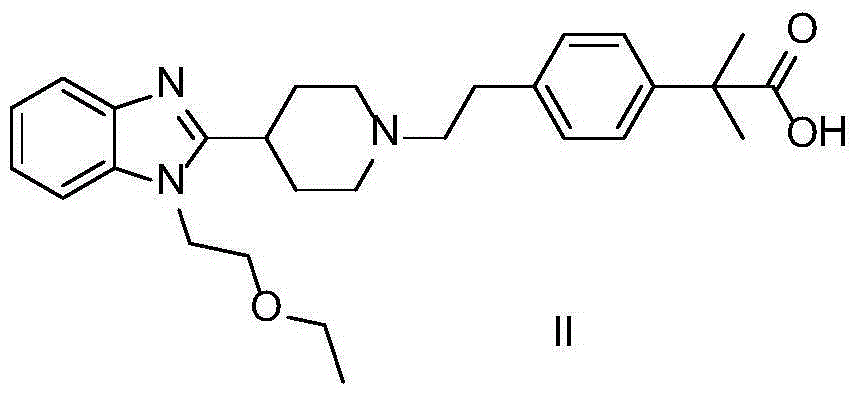
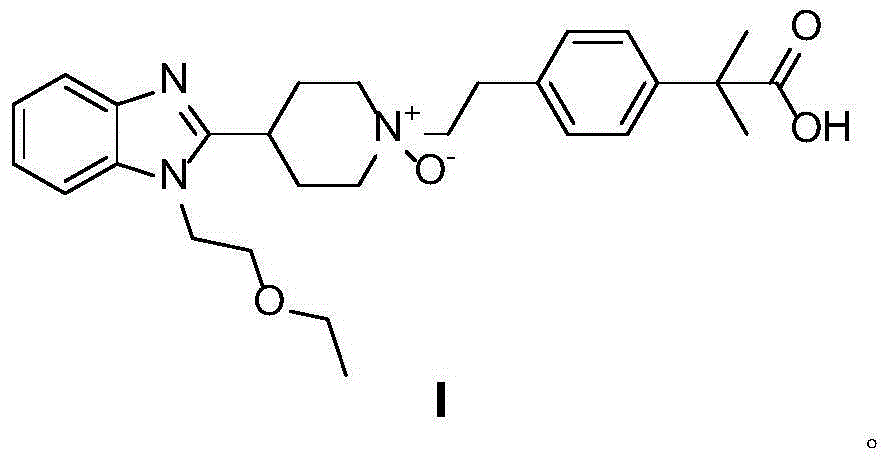
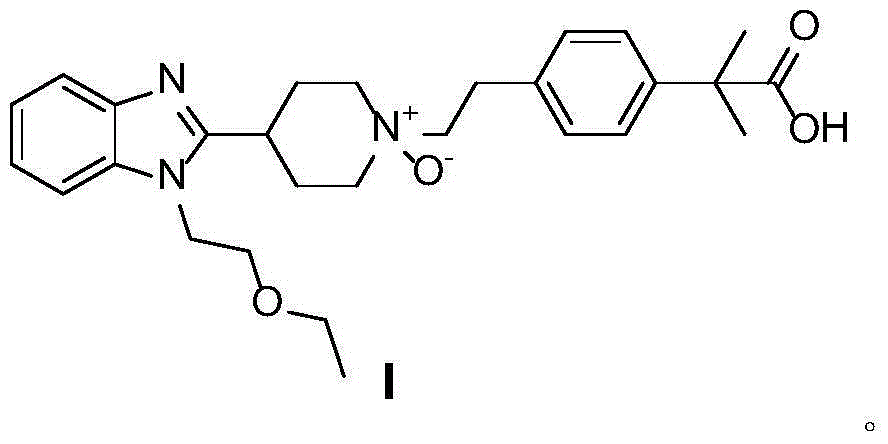
Preparation method of bilastine oxide impurity
A technology for oxidizing impurities and bilastine, which is applied in the direction of organic chemistry, can solve problems affecting product quality and difficult removal, and achieve the effect of simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 10g bilastine 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α- Dissolve dimethylphenylacetic acid in 80mL n-butanol, heat and stir until dissolved, add 12g hydrogen peroxide aqueous solution (30%) dropwise, after the dropwise addition, reflux for 11 hours, cool to room temperature, slowly crystallize, filter , washed, and recrystallized from methanol to obtain 8.7 g of a white solid, with a yield of 84%. 1 H NMR (d 6 -DMSO,500MH z , TMS), δ: 0.9935-1.0203 (t, 3H), 1.4220 (s, 6H), 1.9313-1.9567 (m, 2H), 2.5791-2.6543 (m, 2H), 3.1181 (m, 2H), 3.3400-3.3670 (m,3H),3.4775-3.5239(m,2H),3.6778-3.6859(m,4H),3.8485-3.8690(m,2H),4.4304(t,2H),7.0378-7.0528(m,2H),7.1744 -7.2034 (m, 2H), 7.2934-7.3086 (m, 2H), 7.5319-7.5959 (dd, 2H).
Embodiment 2
[0020] 10g bilastine 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α- Dissolve dimethylphenylacetic acid in 80mL of isopropanol, heat and stir until dissolved, add 11.2g of m-chloroperoxybenzoic acid in batches, reflux for 10 hours, cool to room temperature, slowly crystallize, filter, wash, and reconstitute with methanol After crystallization, 8 g of white solids were obtained with a yield of 78%. 1 H NMR (d 6 -DMSO,500MH z , TMS), δ: 0.9935-1.0203 (t, 3H), 1.4220 (s, 6H), 1.9313-1.9567 (m, 2H), 2.5791-2.6543 (m, 2H), 3.1181 (m, 2H), 3.3400-3.3670 (m,3H),3.4775-3.5239(m,2H),3.6778-3.6859(m,4H),3.8485-3.8690(m,2H),4.4304(t,2H),7.0378-7.0528(m,2H),7.1744 -7.2034(m,2H),7.2934-7.3086(m,2H),7.5319-7.5959(dd,2H)
Embodiment 3
[0022] 10g bilastine 4-[2-[4-[1-(2-ethoxyethyl)-1H-benzimidazol-2-yl]-1-piperidine]ethyl]-α,α- Dissolve dimethylphenylacetic acid in 80mL of n-butanol, heat and stir until dissolved, add 11g of manganese dioxide in batches, reflux for 9 hours, filter while hot, slowly crystallize the filtrate, wash, and obtain a white solid after methanol recrystallization 8.3 g, 80% yield. 1 H NMR (d 6 -DMSO,500MH z , TMS), δ: 0.9935-1.0203 (t, 3H), 1.4220 (s, 6H), 1.9313-1.9567 (m, 2H), 2.5791-2.6543 (m, 2H), 3.1181 (m, 2H), 3.3400-3.3670 (m,3H),3.4775-3.5239(m,2H),3.6778-3.6859(m,4H),3.8485-3.8690(m,2H),4.4304(t,2H),7.0378-7.0528(m,2H),7.1744 -7.2034(m,2H),7.2934-7.3086(m,2H),7.5319-7.5959(dd,2H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com