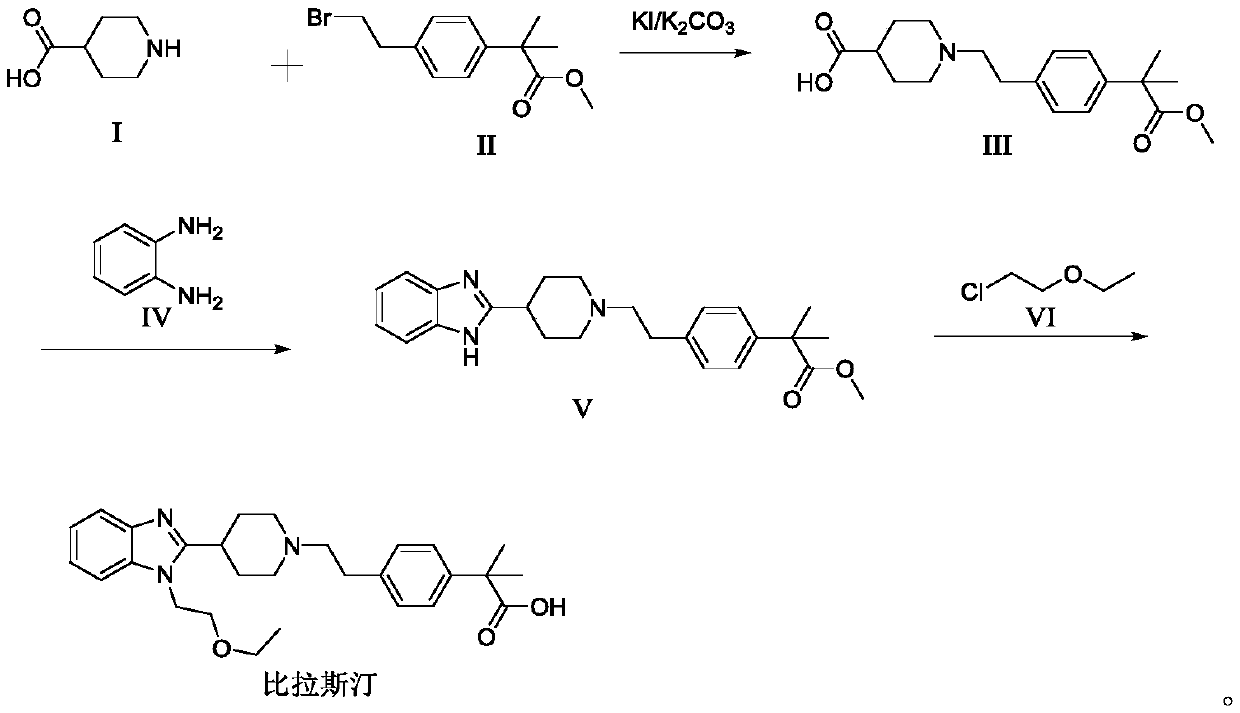
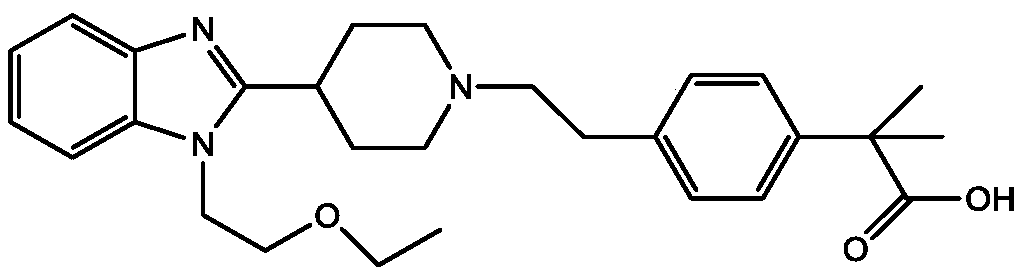
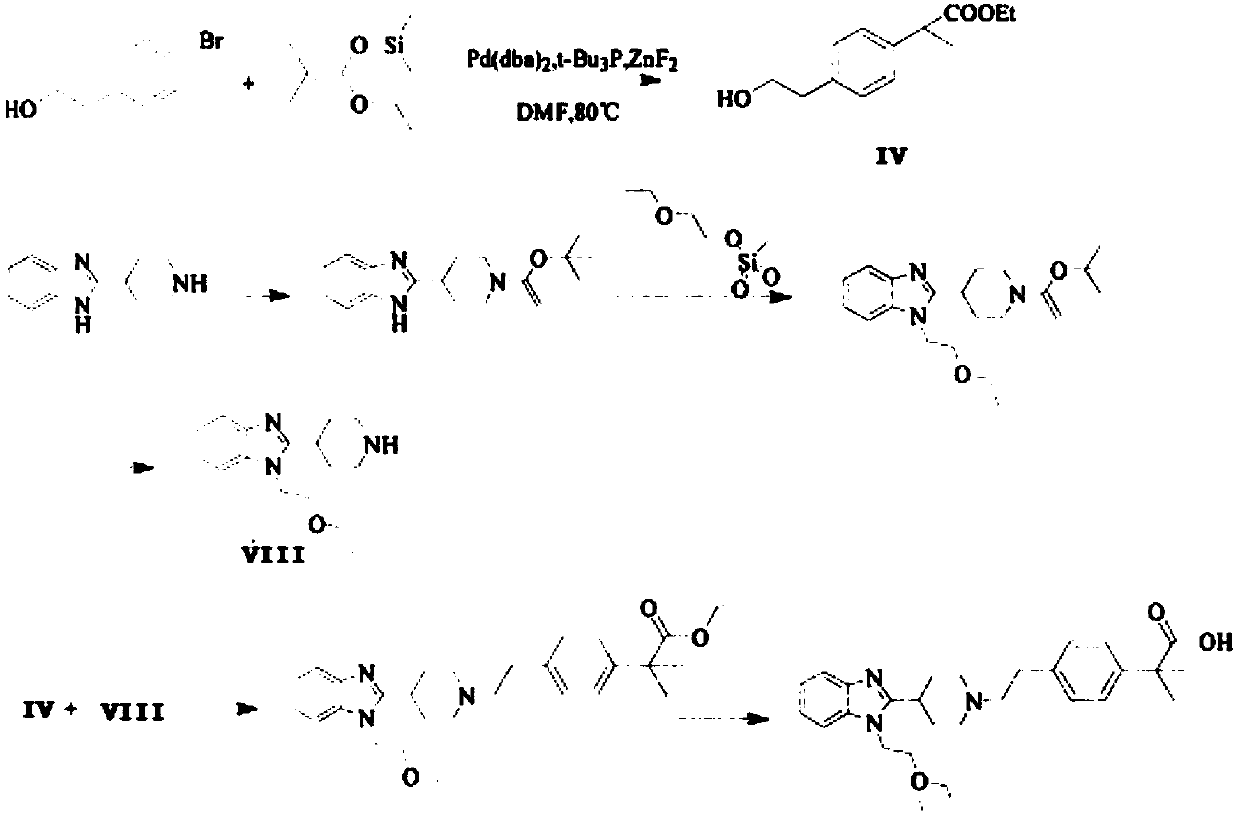
Preparation method of bilastine
A bilastine, molar ratio technology, applied in the field of medicine, can solve the problems of expensive raw materials, reduced industrial production efficiency, low purity and yield, etc., to achieve mild reaction conditions, improve product purity, and good catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example
[0041] Reference example: Preparation of methyl α,α-dimethyl-4-(2-bromoethyl)phenylacetate (Compound II)
[0042] According to the literature [Kong Hao, Geng Haiming, Mei Yudan, etc. Synthesis of bilastine [J]. Chinese Journal of Pharmaceutical Industry, 2015, 46(7): 677-679.] Compound II was prepared:
[0043] Dissolve α,α-dimethyl-phenylacetate methyl ester (5.0g, 28mmol) and bromoacetyl bromide (7.4g, 36mmol) in dichloromethane (20mL), and drop the solution into trichloride at -30°C In a dichloromethane solution (50 mL) of aluminum (11.2 g, 84 mmol), the mixture was dropped and stirred at 0° C. overnight. Add dichloromethane (50ml) to dilute, the reaction solution is filtered through Celite, the filtrate is washed with saturated sodium chloride solution (30ml×2), dried with anhydrous sodium sulfate and filtered, the filtrate is concentrated to obtain a yellow oily α,α -Dimethyl-4-(2-bromoacetyl)phenylacetate methyl ester.
[0044] Add α,α-dimethyl-4-(2-bromoacetyl)phenylacetate ...
Embodiment 1
[0045] Example 1 Preparation of methyl α,α-dimethyl-4-[2-[4-carboxylic acid piperidinyl]ethyl]phenylacetate (Compound III)
[0046] Add 60 mL of N-methylpyrrolidone to a 500 mL reaction flask, add 5.68 g 4-piperidine carboxylic acid (compound I) while stirring, add 6.07 g potassium carbonate, 1.14 g potassium iodide, slowly add 11.41 g compound II, and again add 20 mL N- Methyl-2-pyrrolidone, the temperature was controlled at 40~50℃, the reaction was monitored by TLC after 3h, and the reaction was over after 3h. There was almost no compound II left, filtered, and the filtrate was added with 120mL of purified water at room temperature and stirred. A large amount of solid precipitated out. Continue stirring for 30min. Filter and vacuum dry the filter cake at 50°C for 2h to obtain 11.23g of methyl α,α-dimethyl-4-[2-[4-carboxylate piperidinyl]ethyl]phenylacetate, yield 84.2%, purity 98.6 %.
Embodiment 2
[0047] Example 2 Preparation of methyl α,α-dimethyl-4-[2-[4-carboxylate piperidinyl]ethyl]phenylacetate (Compound III)
[0048] Add 60 mL of N-methylpyrrolidone to the 500mL reaction flask, add 5.68g of compound I while stirring, add 7.18g of potassium carbonate, 1.14g of potassium iodide, slowly add 11.41g of compound II, and again add 20mL of N-methyl-2-pyrrolidone, control React at 40~50℃. After 3h, the reaction is monitored by TLC. There is almost no compound II left. Filter. Add 120mL of purified water to the filtrate at room temperature and stir. A large amount of solid precipitates. Continue stirring for 30min and filter. The filter cake is vacuum at 50℃. After drying for 2 hours, 11.95 g of methyl α,α-dimethyl-4-[2-[4-carboxylate piperidinyl]ethyl]phenylacetate was obtained, with a yield of 89.6% and a purity of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com