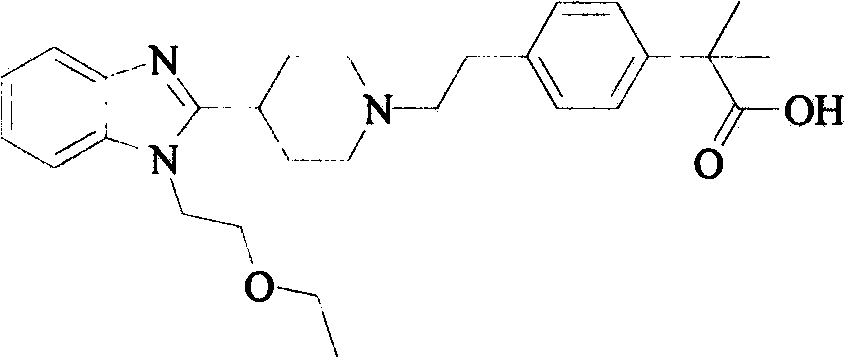
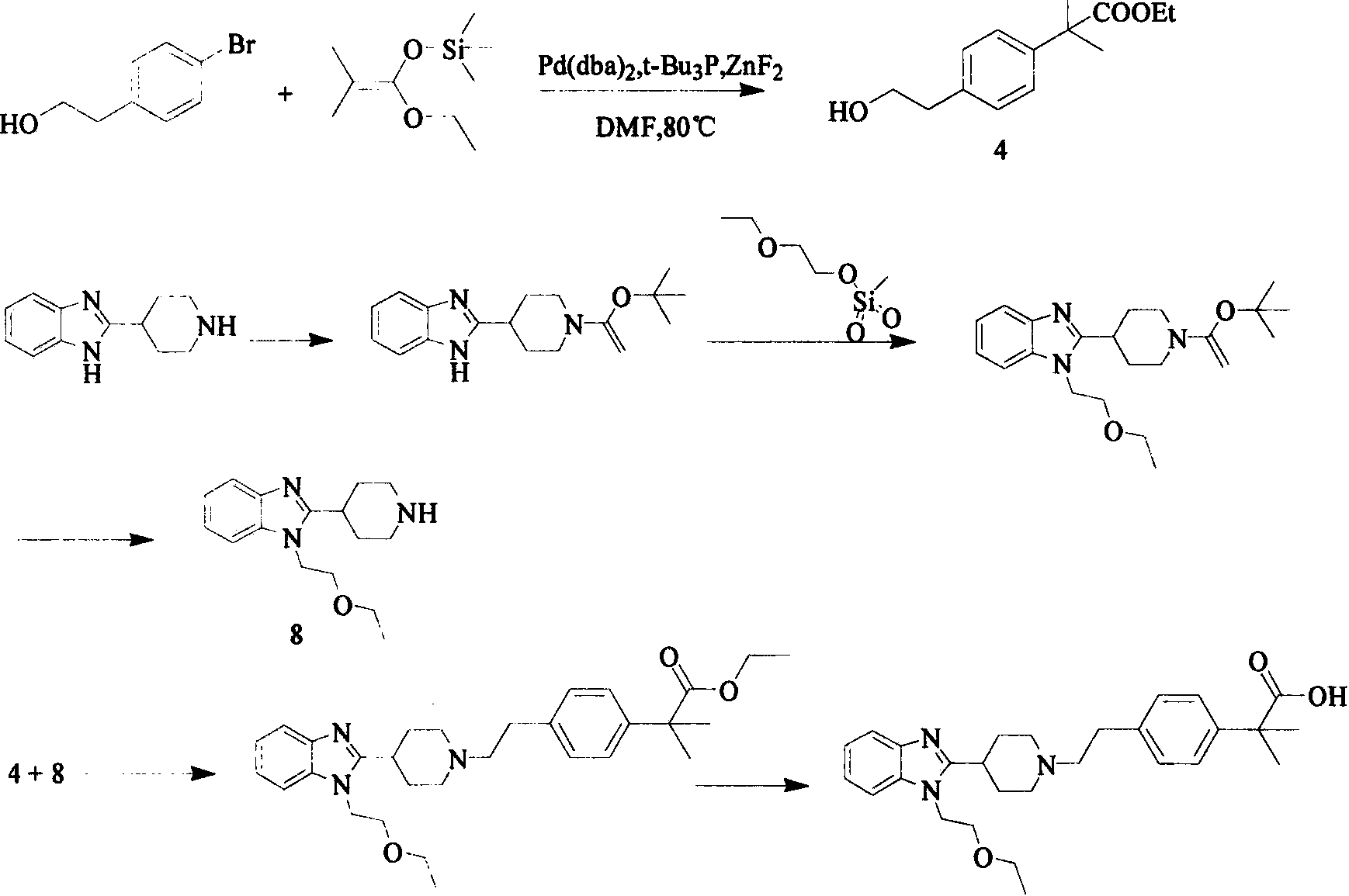
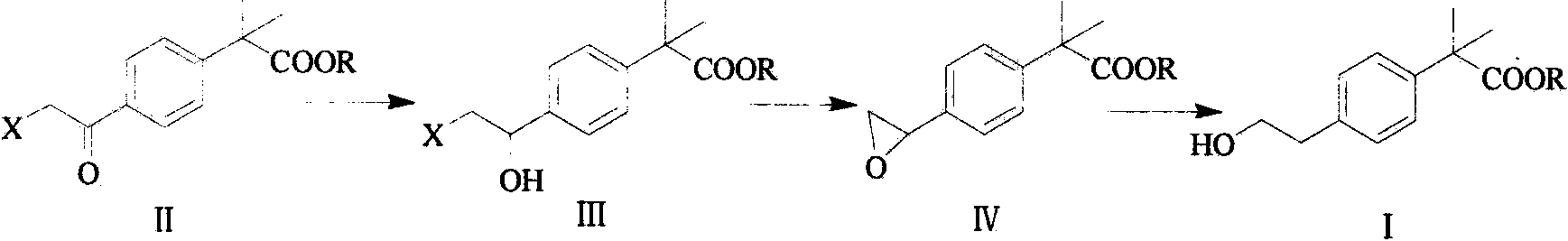Preparation method of bilastine key intermediate
A bilastine and intermediate technology, applied in the field of pharmaceutical preparation, can solve the problems of difficult operation, difficult industrial production, large pollution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: 2, the synthesis of ethyl 2-dimethylphenylacetate
[0028] Add 2,2-dimethylphenylacetic acid (500g, 3.1mol) and 2.5L dichloromethane solution into a 10L reaction flask, stir at room temperature, add thionyl chloride (750g, 6.3mol), and heat up to system reflux React for 15 hours in the state, then slowly add 400mL of absolute ethanol dropwise and stir for a period of time. After the reaction is detected by TLC, adjust the pH value to 9-10 with NaOH solution, separate the organic layer, wash twice with saturated sodium bicarbonate, and then wash with saturated chlorine After washing twice with sodium chloride, it was concentrated and dried to obtain ethyl 2,2-dimethylphenylacetate (560 g, 96%).
Embodiment 2
[0029] Embodiment 2: the synthesis of ethyl 2-(4-chloroacetyl) phenyl-2-methyl propionate
[0030] In a 5L reaction flask, add ethyl 2,2-dimethylphenylacetate (542g, 2.8mol), 2L dichloromethane solution, stir, add anhydrous aluminum chloride (600g, 4.5mol), and drop Add 500mL dichloromethane mixed solution containing chloroacetyl chloride (318.6g, 2.8mol), and then react for a period of time, stop the reaction after the completion of the TLC detection reaction, separate the organic layer, and use water and saturated sodium bicarbonate solution respectively for the organic layer , saturated sodium chloride solution, concentrated and dried to obtain ethyl 2-(4-chloroacetyl)phenyl-2-methylpropanoate (660 g, 87%).
Embodiment 3
[0031] Embodiment 3: the synthesis of ethyl 2-(4-bromoacetyl)phenyl-2-methylpropionate
[0032] According to the operation of Example 2, the chloroacetyl chloride in Example 2 was replaced with bromoacetyl bromide to obtain ethyl 2-(4-bromoacetyl)phenyl-2-methylpropionate (yellow liquid, 86%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com