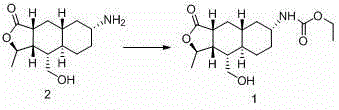
Preparation methods of himbacine analogue and intermediate thereof
A compound and a conversion technology, which are applied in the field of preparation of himbacine analogs and their intermediates, can solve the problems of unsuitability for industrial production, low yield and high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
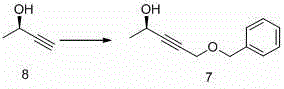
[0119] Example 1 : Preparation of compound 7
[0120]
[0121] Add 60 mL of tetrahydrofuran and 10 μL of concentrated sulfuric acid to a 1L three-necked flask in sequence, and then add the compound while stirring. 8 (10 g, 0.14 mol) and hexamethyldisilazane (11.5 g, 0.07 mol) were replaced by nitrogen three times, and heated to 60° C. for 3 h under reflux. Slowly cool the reaction solution to -40°C and add 272mL of tetrahydrofuran, add dropwise n-butyllithium (2.5M in n-hexane, 68.5mL, 0.17mol) at a temperature below -30°C, and then control the temperature at -30°C Next, a solution of chloromethyl benzyl ether (26.8 g, 0.17 mol) in toluene (110 mL) was added dropwise, and stirred for 1 h after the drop was completed. Quench the reaction with water (20 mL), filter, wash the filter cake with ethyl acetate and add to the filtrate, pull the filtrate to dryness and purify by column chromatography to obtain 20.4 g of light yellow liquid, yield: 75.25%. 1 HNMR (400MHz, CDCl 3...
Embodiment 2
[0122] Example 2 : Preparation of compound 6
[0123]
[0124] Add compounds sequentially to a 500mL three-necked bottle 7 (19g, 0.1mol), Lindlar catalyst (5%Pb poisoning, 1.9g5%Pd / CaCO 3 , 0.018mol) and ethyl acetate 380mL, after nitrogen replacement three times and hydrogen replacement three times, stir at 25°C under normal pressure for 5h. The catalyst was removed by filtration, the filter cake was washed with ethyl acetate and then added to the filtrate, and the filtrate was pulled to dryness to obtain 18.4 g of light yellow liquid, yield: 95.83%. MS[M+H] + :193.
Embodiment 3
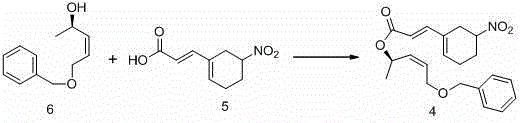
[0125] Example 3: Preparation of Compound 4
[0126]
[0127] In the 250mL three-necked bottle, add the compound in sequence 5 (10g, 0.05mol) and 50mL of toluene, the suspension was cooled to 0°C, and slowly added N-methylmorpholine (10.5g, 0.10mol) and trimethylacetyl chloride (6g, 0.05mol ) in toluene (25mL), and stirred at 0°C for 1h after the drop was completed. Slowly add compound under temperature control below 5°C 6 (9.6g, 0.05mol) in toluene (40mL) and tetrahydrofuran (22mL) solution, then add 4-dimethylaminopyridine (0.61g, 0.005mol) in tetrahydrofuran (6mL) solution, stir the mixture at about 0°C for 12h . After the reaction is complete, add 4NH 2 SO 4 (25mL) solution to quench the reaction, raise the temperature to no more than 25°C and stir for 1h, filter, wash the filter cake three times with ethyl acetate, separate the layers, extract the aqueous layer with ethyl acetate (2*25mL) and merge it into the organic layer, organic layer by layer with 5% K 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com