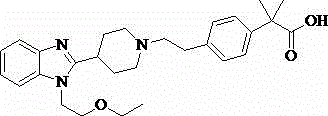
Method for preparing novel crystal form of bilastine
A bilastine and crystal form technology, applied in the field of medicinal chemistry, can solve problems such as instability of crystal form II and crystal form III
Inactive Publication Date: 2014-11-19
BEIJING VENTUREPHARM BIOTECH
View PDF6 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
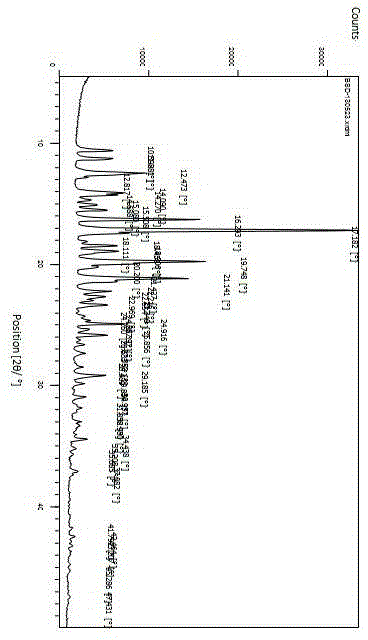
[0004] In the Chinese patent CN1628112A, the crystal form of bilastine was first proposed. Crystal forms I, II, III, crystal form II and crystal form III are unstable and can be converted into crystal form I. The patent only gives the unit cell data and infrared data
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0014] Add 3g of bilastine to the reaction flask, add 18ml of acetone and water mixed solvent (90:10), heat until bilastine dissolves, cool to room temperature to crystallize, filter and dry to obtain the new crystal form of bilastine , yield 80.1%.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a method for preparing a novel crystal form of bilastine. The method comprises the following steps: dissolving bilastine in a mixed solvent of an organic solvent and water, heating until all bilastine is dissolved, and cooling and crystallizing to obtain the novel crystal form of bilastine.
Description
technical field [0001] The invention belongs to the field of medicinal chemistry and relates to 2-[4-(2-{4-[1-(2-ethoxy-ethyl)-1H-benzimidazol-2-yl]-piperidin-1-yl }Ethyl)-phenyl]-2-methyl-propionic acid (bilastine) new crystal preparation. Background technique [0002] Bilastine, the 2nd generation histamine H developed for Spanish FAES pharmaceutical company 1 Receptor antagonist, approved by the European Union for the treatment of allergic rhinitis and chronic idiopathic urticaria in 2010, and launched in Ireland in 2011. This product is safe, without the sedative effect and cardiotoxicity of commonly used antihistamines. [0003] [0004] In the Chinese patent CN1628112A, the crystal form of bilastine was first proposed. Crystal forms I, II, III, crystal form II and crystal form III are unstable and can be converted into crystal form I. The patent only gives the unit cell data and infrared data. In the patent CN201310107513.8, a new crystal form of bilastine is men...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D401/04A61K31/454A61P11/02A61P37/08A61P17/00
CPCC07D401/04C07B2200/13
Inventor 赵月楠闫起强马苏峰
Owner BEIJING VENTUREPHARM BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com