Bilastine detection method
A detection method and a bilastine technology, which are applied in the field of analytical chemistry, can solve the problems that bilastine does not affect the efficacy of bilastine raw materials and its preparations, and achieve the effect of improving accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Sample preparation
[0040] Take bilastine, formula (II), formula (III), formula (IV), formula (V), formula (VI), formula (VII) and bilastine oxidation damage samples respectively, add acetonitrile and water volume Acetonitrile aqueous solution with a ratio of 50:50 was used to make a mixed solution containing about 0.06 mg / mL of bilastine and about 0.002 mg / mL of each impurity monomer, which was the sample to be tested.
[0041] Wherein, the bilastine damaged sample was obtained by mixing 20 mg of bilastine with 2 mL of 30% hydrogen peroxide and reacting at 50° C. for 30 minutes.
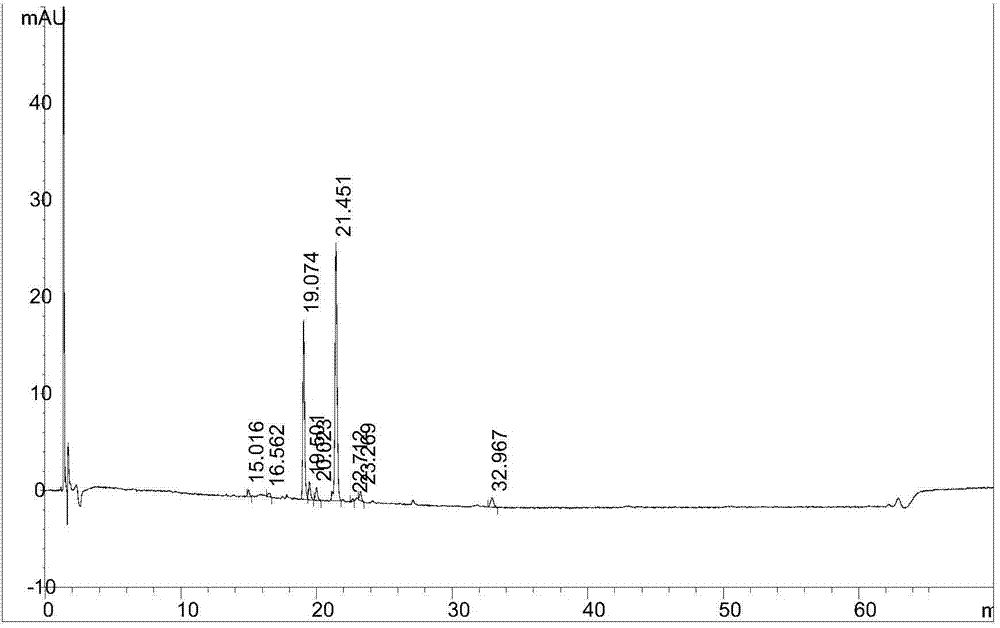
[0042] The oxidative damage sample solution and the mixed solution of other impurities are mixed again for chromatographic analysis, and the chromatographic analysis is carried out immediately for the sample to be tested. For the results, see figure 1 , figure 1 It is the HPLC collection of illustrative plates that according to the test method described in the embodiment of the present inv...
Embodiment 2
[0059] Sample preparation
[0060] Take the bilastine bulk drug and add acetonitrile and water with a volume ratio of 50:50 in an acetonitrile aqueous solution to make a solution containing about 2 mg / mL of bilastine, which is the sample to be tested.
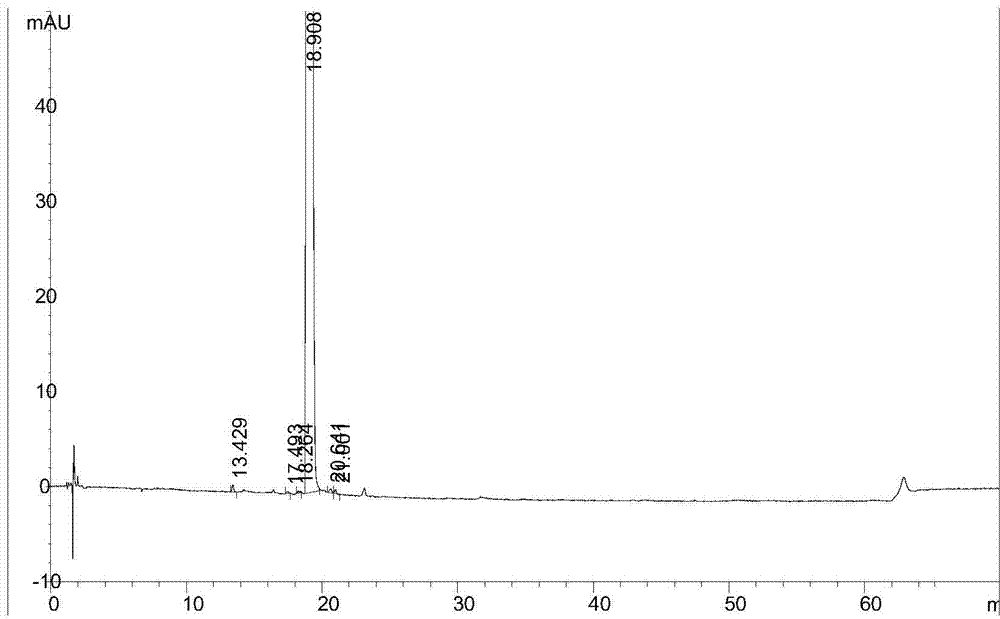
[0061] The obtained sample to be tested is subjected to chromatographic analysis, and the results are shown in image 3 , image 3 It is the HPLC collection of illustrative plates that according to the test method described in the embodiment of the present invention 2, the sample to be tested described in the embodiment 2 is detected;
[0062] Instrument: Agilent 1100 (or 1200) high performance liquid chromatography and its workstation VWD (or DAD) detector
[0063] Chromatographic column: Agilent Eclipse XDB-C184.6×150mm, 5μm
[0064] Mobile phase A: 0.01mol / L ammonium acetate buffer (containing 0.1% triethylamine, 0.05% sodium heptanesulfonate, adjust pH to 5.0 with acetic acid)
[0065] Mobile Phase B: Acetonitrile
[0...
Embodiment 3
[0076] Sample preparation
[0077] Take an appropriate amount of bilastine preparation fine powder, approximately equivalent to 20mg of bilastine, put it in a 10mL measuring bottle, add an appropriate amount of acetonitrile aqueous solution (diluent) with a volume ratio of acetonitrile and water of 50:50 and sonicate for 15min, then add the diluent Dilute to the mark, shake well, filter, and take the filtrate as the sample to be tested.
[0078] Prepare the blank excipient solution in the same way to obtain the blank control sample.
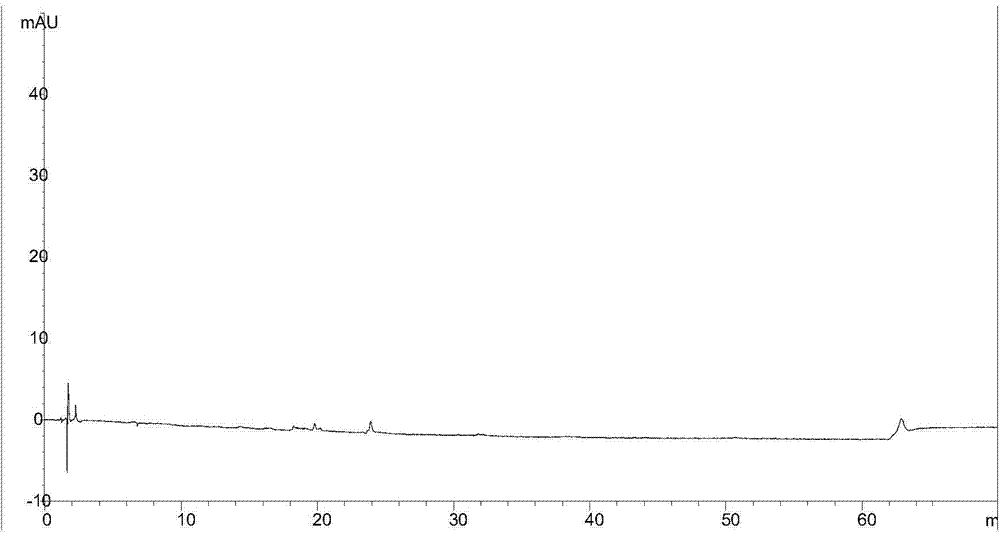
[0079] The obtained sample to be tested is subjected to chromatographic analysis, and the results are shown in Figure 4 , Figure 4 For according to the test method described in the embodiment of the present invention 3 to the HPLC collection of illustrative plates of the sample to be tested described in embodiment 3;
[0080] The blank control sample was carried out chromatographic analysis, the results can be found in Figure 5 , Figure ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com