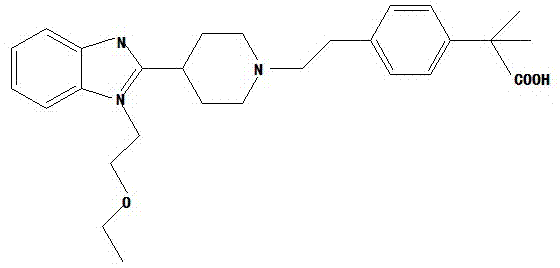
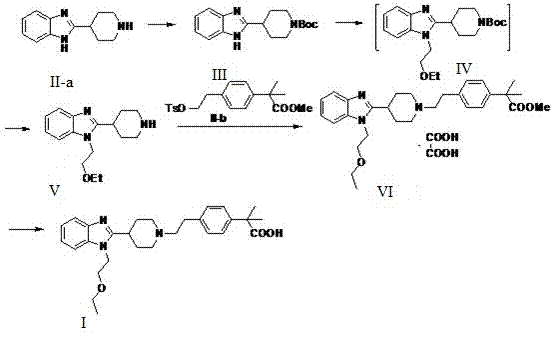
Bilastine crystal form and preparation method thereof
A crystal form and drug technology, applied in the field of drug preparation, can solve problems such as undisclosed crystal form information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
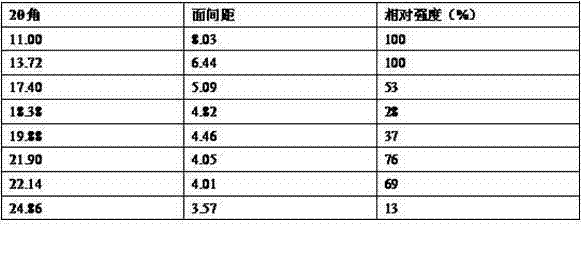
[0018] Put an appropriate amount of bilastine into 100 times the volume of isopropanol, and heat to reflux for about 15-20 minutes while stirring under a nitrogen atmosphere. Cool slowly, and cool the reaction liquid to about 50°C over 6 hours, and stop stirring. Cool the reaction solution to room temperature, stir again for 3 hours, filter, wash the solid with a small amount of cold isopropanol, and dry it in vacuum at 35-40°C to constant weight to obtain a stable crystal form of bilastine, the obtained bilastine X-ray powder diffraction 2θ (±0.2) data are: 11.30, 12.50, 17.18, 18.94, 19.80, 21.14, 22.68, 24.92.
[0019]
Embodiment 2
[0021] Put 830g of bilastine crude product and 83L of isopropanol into a 100L reactor, protect it with nitrogen, heat to reflux, dissolve and clarify, filter out the insoluble matter while it is hot, cool the filtrate to room temperature, stir and crystallize for 10-12h, and filter with suction. The filter cake was washed with a small amount of isopropanol, dried in vacuum at 50-60°C to constant weight, about 45-50h, and 610-660g of white solid was obtained, and a stable crystal form of bilastine was obtained, and the obtained bilastine X- X-ray powder diffraction 2θ (±0.2) data are: 11.30, 12.50, 17.18, 18.94, 19.80, 21.14, 22.68, 24.92.
Embodiment 3
[0023] Put an appropriate amount of bilastine into 100 times the volume of methanol ethyl acetate (1:1 mixture), and heat to reflux for about 15-20 minutes while stirring under a nitrogen atmosphere. Cool slowly, and cool the reaction liquid to about 50°C over 6 hours, and stop stirring. The reaction solution was cooled to room temperature, stirred again for 3 hours, filtered, washed with a small amount of cold ethyl acetate, dried in vacuum at 35-40°C to constant weight, and the yield was calculated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com