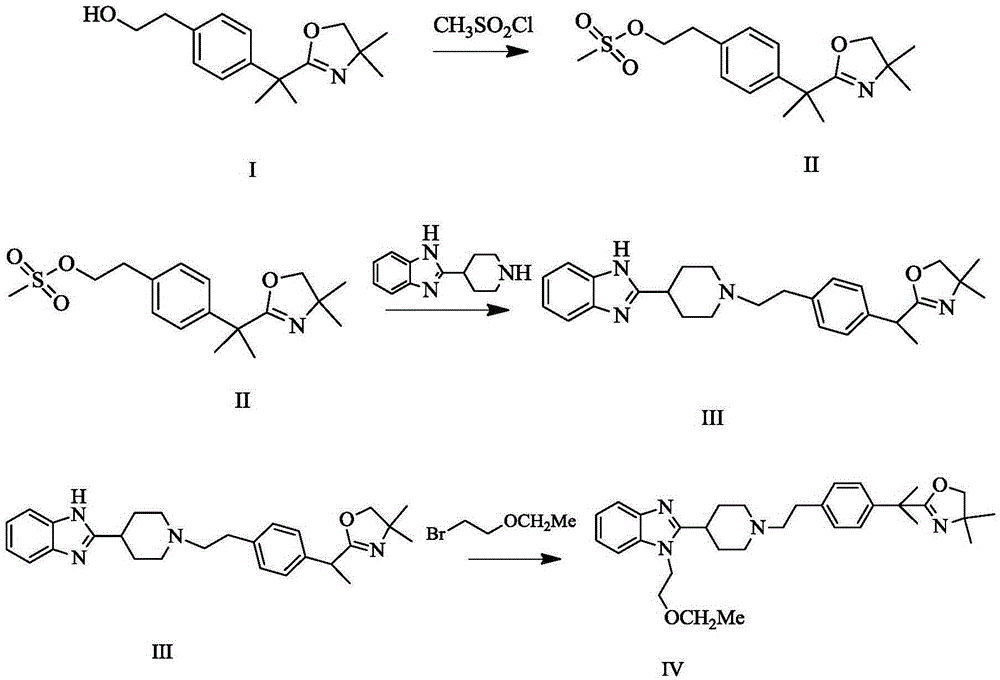
Bilastine intermediate preparation method
A technology of bilastine and intermediates, applied in the field of medicinal chemistry, can solve the problems of high requirements for reaction equipment, difficulty in industrialization, and low reaction yield, and achieve the effects of high purity, no side reactions, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
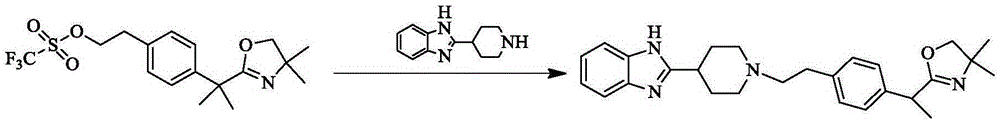
[0015] Dissolve 9.70 g of the compound 2-{1-[4-(2-hydroxyethyl)phenyl]-1-methylethyl}-4,5-2H-4,4-dimethyloxazole in dichloro In 50mL of methane, turn on the magnetic stirring, then dissolve 14.2g of trifluoromethanesulfonyl chloride in 10ml of dichloromethane, put it in a constant pressure dropping funnel, drop it into the reaction bottle, then add 8mL of triethylamine, and react at room temperature for 4h , TLC point plate monitoring shows that the raw material has reacted completely, and the saturated NaHCO 3 solution, water and NaCl solution extraction, the organic phase is concentrated into a yellow oily liquid, add an appropriate amount of ethyl acetate to dissolve it under heating, then add petroleum ether for beating until a large amount of solids are precipitated, freeze and filter (increase the yield ), dried to obtain 11.4 g of white powdery solid, (single point) yield was 86.1%.
[0016] Take 2-[4-(1-(4,4-Dimethyl-2H-oxazol-2-yl)-1-methylethyl)phenyl]ethyl triflate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com