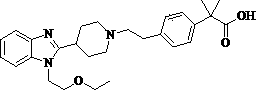
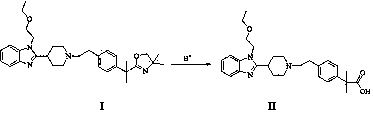Preparation method of Bilastine
A raw material, aqueous solution technology, applied in organic chemistry and other directions, can solve the problems of ether bond cleavage, difficult removal of impurities, difficult product purification, etc., and achieves the effects of mild reaction conditions, no side reactions, and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
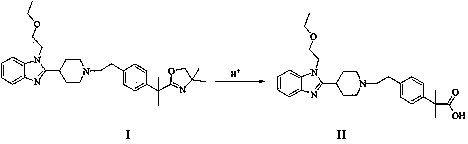
[0019] 5g 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-1-methyl-ethyl]-benzene Base}-ethyl)-piperidin-4-yl]-1-(2-ethoxy-ethyl)-1H-benzimidazole was added to a clean three-neck flask, 150ml of 4N formic acid solution was added, and heated to reflux for 36 hours , adjusted to pH=7 with 10% aqueous sodium hydroxide solution, extracted with dichloromethane, and the solvent was spinned out to obtain a white solid with a yield of 90.5%.
Embodiment 2
[0021] 5g 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-1-methyl-ethyl]-benzene Base}-ethyl)-piperidin-4-yl]-1-(2-ethoxy-ethyl)-1H-benzimidazole was added to a clean three-necked flask, 150ml of 4N trifluoroformic acid solution was added, and heated to reflux After 36 hours, adjust the pH to 7 with 10% aqueous sodium hydroxide solution, extract with dichloromethane, and spin out the solvent to obtain a white solid with a yield of 92.6%.
Embodiment 3
[0023] 5g 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-dihydro-oxazol-2-yl)-1-methyl-ethyl]-benzene Base}-ethyl)-piperidin-4-yl]-1-(2-ethoxy-ethyl)-1H-benzimidazole was added to a clean three-neck flask, 150ml of 4N acetic acid solution was added, and heated to reflux for 36 hours , adjusted to pH=7 with 10% aqueous sodium hydroxide solution, extracted with dichloromethane, and the solvent was spinned out to obtain a white solid with a yield of 89.7%.
[0024]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com