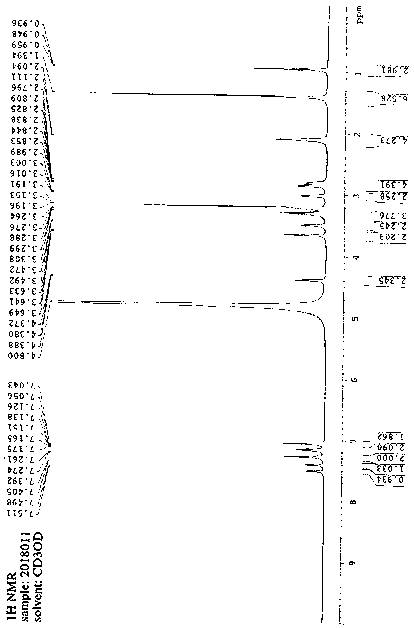
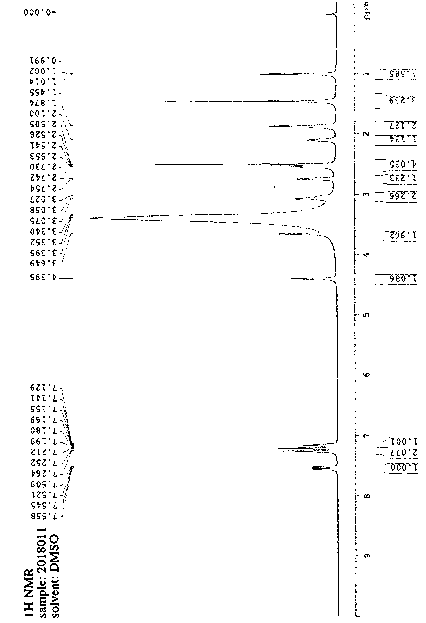
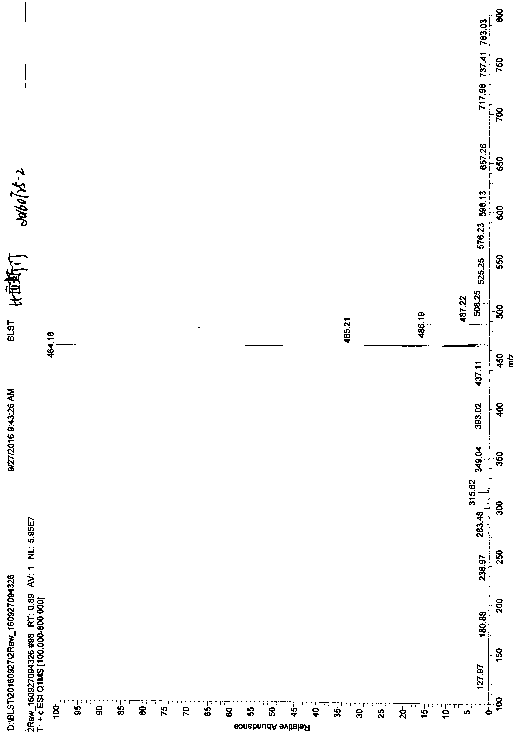
Method for preparing bilastine
A compound and raw material technology, applied in the field of preparation of Bilastine, can solve the problems of ether bond breakage, drug quality decline, solvent increase, etc., and achieve mild reaction conditions, no side reactions, and simple operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0028] A kind of method for preparing bilastine of the present invention comprises the following steps:
[0029] will structure Add the compound into water, cool down to 0-5 degrees Celsius, add alkali, phase transfer catalyst, and p-toluenesulfonyl chloride, stir and condense and filter directly to obtain the structure compound of. The base mentioned therein includes sodium hydroxide, sodium hydroxide, potassium hydroxide, magnesium hydroxide, lithium hydroxide, barium hydroxide, sodium methoxide, potassium methoxide, sodium ethoxide, potassium ethoxide or potassium tert-butoxide, wherein hydrogen Sodium oxide or potassium hydroxide; phase transfer catalysts include tetrabutylammonium chloride, tetrabutylammonium bromide, tetrabutylammonium iodide, benzyltriethylammonium chloride, tetrabutylammonium hydrogensulfate, trioctylammonium Methyl Ammonium Chloride, Dodecyl Trimethyl Ammonium Chloride, Tetradecyl Trimethyl Ammonium Chloride, Polyethylene Glycol Dimethyl Ether, Po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com