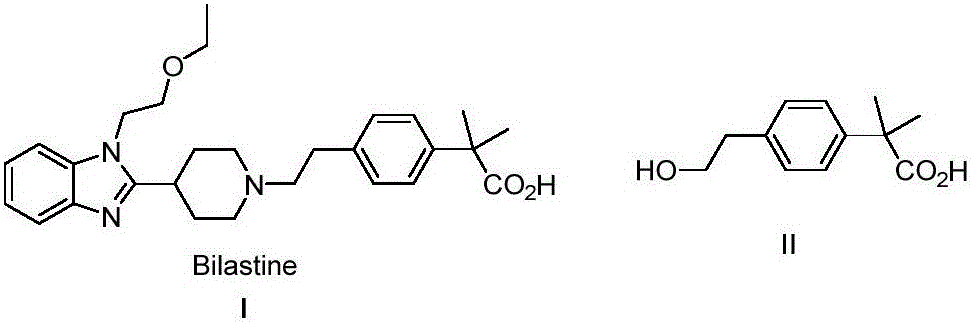
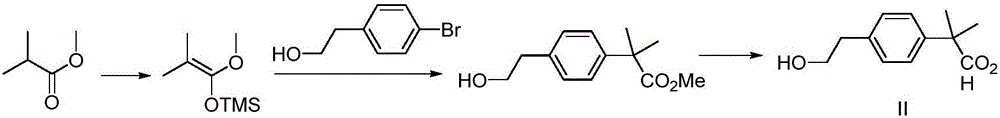
Preparation method of new anti-allergic medicine Bilastine intermediate
A bilastine and intermediate technology, which is applied in the field of preparation of bilastine intermediates of new antiallergic drugs, can solve the problems of unstable enol silyl ether construction, decreased yield, high price and the like, and achieves low synthesis cost. , the effect of mild reaction conditions and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
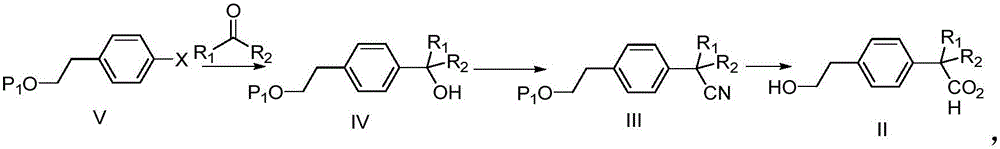
[0030] The preparation method steps are as follows:
[0031] (1) compound V is added to obtain compound IV after halogen carbanion exchange;
[0032] (2) compound IV obtains compound III through electrophilic cyanation reaction;
[0033] (3) Compound II is obtained after compound III is hydrolyzed.
[0034] In a particularly preferred scheme, the present invention provides the following synthetic route:
[0035]
[0036] This synthetic method comprises the following steps:
[0037] 1) compound shown in formula Va, after carbanion exchange, addition obtains compound shown in formula IVa;
[0038] 2) Compounds shown in formula IVa, electrophilic cyanation to obtain compounds shown in formula IIIa;
[0039] 3) The compound shown in formula IIIa is hydrolyzed to obtain the compound shown in formula II.
[0040] The terms used in the present invention have the following meanings unless otherwise stated.
[0041] "Alkyl" refers to a saturated aliphatic hydrocarbon group, in...
Embodiment
[0044] 1: Synthesis of Compound Va
[0045] Add 4-bromophenylethanol (1.0kg, 5.0mol) to the reaction kettle, dissolve 10 times the volume of dichloromethane, add triethylamine (1.01kg, 10.0mol) and a catalytic amount of DMAP, add TBSCl (0.9kg ,6.0mol). React at room temperature until the raw materials disappear, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate to give compound Va (1.5kg, 95.5%) as a colorless oily liquid, which is directly put into the next reaction.
[0046] 2: Synthesis of Compound IVa
[0047] Add the above compound Va (1.5kg, 4.78mol) into the reaction kettle, dissolve in anhydrous THF (10V), cool to -78°C, slowly add n-butyllithium (2.5M, 2.3L) dropwise, after the dropwise addition is completed, keep This temperature was reacted for 2 hours. Anhydrous acetone (0.55 kg) was slowly added dropwise over 1 hour. After the dropwise addition is completed, slowly rise to room temperature and react until the raw ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com