Preparation method of bilastine
A technology of bilastine and molar ratio, which is applied in the field of preparation of the compound of formula I of bilastine raw material medicine, and can solve the problem of generating quaternary ammonium salt by-products and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
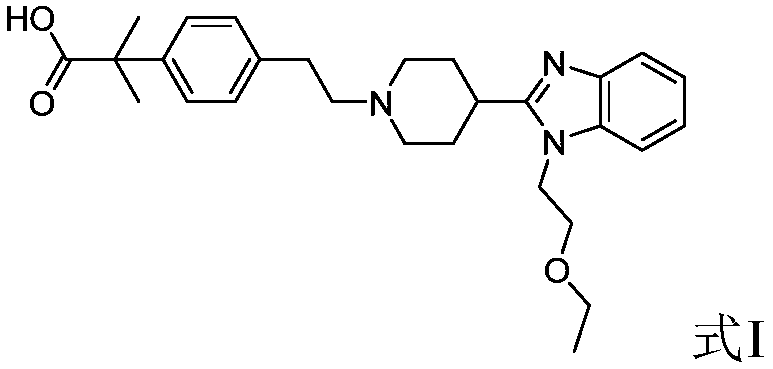
[0054] The preparation method of bilastine,
[0055] The present invention provides a kind of preparation method of bilastine, described method comprises steps:
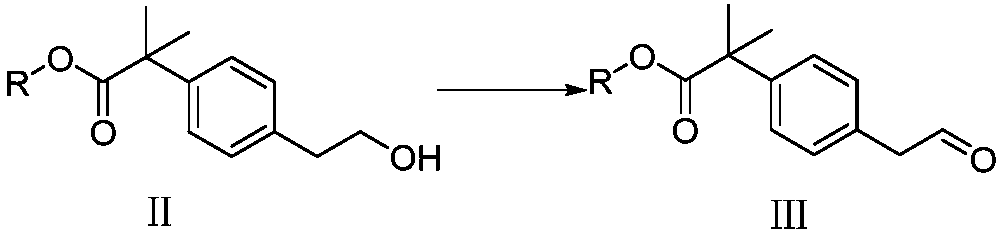
[0056] (1) Formula II compound (for example, methyl 4-hydroxyethylphenyl tert-butyrate) is dissolved in the first solvent, adding oxidizing agent (for example, 2,2,6,6-tetramethylpiperidine oxide ( TEMPO) and sodium hypochlorite aqueous solution), oxidation to obtain the compound of formula III (such as, 4-acetaldehyde methyl phenyl tert-butyrate);
[0057]
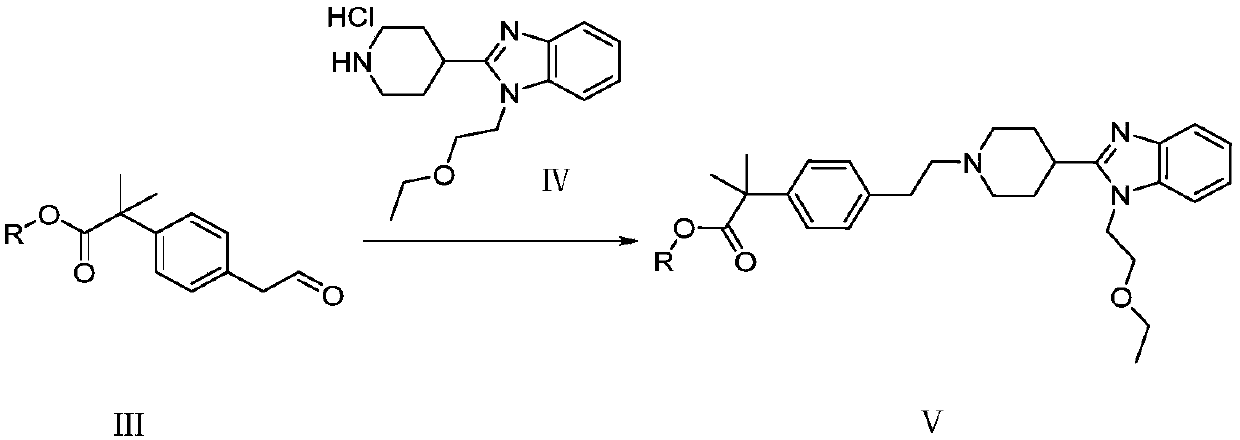
[0058] (2) Formula III compound and 1-(2-ethoxyl-ethyl)-2-piperidin-4-yl-1H-benzimidazole hydrochloride (formula IV) are dissolved in the second solvent, under stirring React for a period of time (such as 1 hour), add a reducing agent (for example, sodium borohydride), and reductively aminate to obtain 4-[2-[1-(2-ethoxyethyl) benzimidazolyl] piperidinyl] Ethyl-phenylmethyl tert-butyrate (Formula V).
[0059]
[0060] (3) 4-[2-[1-(2-ethoxyethyl) benzimid...
Embodiment 1
[0076] 4-Hydroxyethylphenyl methyl tert-butyrate (222g) was dissolved in dichloromethane (1L), added 2,2,6,6-tetramethylpiperidine oxide (TEMPO) (1g), cooled to 0°C, add sodium hypochlorite aqueous solution (13%, 600mL) dropwise, stir for 1h, carry out post-treatment (i.e. liquid separation, the organic phase is successively washed with 10% aqueous sodium bisulfite solution (1L), saturated brine (1L), and concentrated in vacuo dry), to obtain methyl 4-acetaldehyde phenyl tert-butyrate (185 g), with a purity of 95.3%, and a yield of 83.3%.
Embodiment 2
[0078] Methyl 5-acetaldehyde phenyl tert-butyrate (220g) was dissolved in methanol (1.5L), and 1-(2-ethoxy-ethyl)-2-piperidin-4-yl-1H was added in portions - Benzimidazole hydrochloride (340g), stirred at room temperature for 1 hour, cooled to 0°C, added 10% methanolic sodium hydroxide solution (400mL), added sodium borohydride (76g) in batches, and reacted at room temperature for 2 hours, vacuum Concentrate to remove most of the methanol, add water (1.5L) dichloromethane (1.5L) to the residue, extract and separate the liquids, and concentrate the organic phase in vacuo to obtain 4-[2-[1-(2-ethoxyethyl)benzimidazole Base]piperidinyl]ethyl-phenyl-tert-butyric acid methyl ester (421g), purity: 96.2%; yield: 88.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com