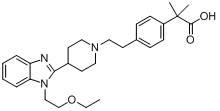
Preparation method of 2-(4-haloethyl) phenyl-2-methyl propionic ester and synthesis method of bilastine
A technology of methyl propionate and haloethyl, which is applied in the field of drug synthesis, can solve problems such as difficult industrial production, difficult operation, and high cost, and achieve the effects of stable raw material properties, easy availability of raw materials, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Synthesis of 2-(4-haloethyl)phenyl-2-methyl propionate
[0061] a. In dichloromethane, 2,2-dimethylphenylacetate and chloroacetyl chloride undergo acylation reaction under the action of aluminum trichloride. The acylation reaction conditions are: temperature is 5°C; time is 3 hours , generating 2-(4-haloacetyl)phenyl-2-methylpropionate,
[0062] b, 2-(4-haloacetyl) phenyl-2-methyl propionate undergoes a Kisner-Wolf-Huang Minglong reduction reaction, reducing the carbonyl to generate 2-(4-haloethyl) phenyl- 2-methyl propionate; the reduction reaction conditions are as follows: the solvent is polyethylene glycol, the reaction temperature is 180° C.; the reaction time is 3 hours.
Embodiment 2
[0063] Example 2 Synthesis of 2-(4-haloethyl)phenyl-2-methyl propionate
[0064] a. In tetrahydrofuran, 2,2-dimethylphenylacetate and bromoacetyl bromide undergo acylation reaction under the action of aluminum trichloride. The acylation reaction conditions are: temperature is 0°C; time is 24 hours, and 2-(4-Haloacetyl)phenyl-2-methylpropionate,
[0065] b, 2-(4-haloacetyl) phenyl-2-methyl propionate undergoes a Kisner-Wolf-Huang Minglong reduction reaction, reducing the carbonyl to generate 2-(4-haloethyl) phenyl- 2-methyl propionate; the reduction reaction conditions are: the solvent is polyethylene glycol, the reaction temperature is 100°C; the reaction time is 8 hours.
Embodiment 3
[0066] Example 3 Synthesis of 2-(4-haloethyl)phenyl-2-methylpropionate
[0067] a. In nitromethane, 2,2-dimethylphenylacetate and chloroacetyl bromide undergo acylation reaction under the action of aluminum trichloride. The acylation reaction conditions are: temperature is 100°C; time is 1 hour , generating 2-(4-haloacetyl)phenyl-2-methylpropionate,
[0068] b, 2-(4-haloacetyl) phenyl-2-methyl propionate undergoes a Kisner-Wolf-Huang Minglong reduction reaction, reducing the carbonyl to generate 2-(4-haloethyl) phenyl- 2-methyl propionate; the reduction reaction conditions are as follows: the solvent is polyethylene glycol, the reaction temperature is 200° C.; the reaction time is 1 hour.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com