Intermediate of Sitagliptin and synthesis method of intermediate
A technology for sitagliptin and a synthesis method, applied in the field of drug synthesis, can solve the problems of potential safety hazards, unfavorable large production, high toxicity of reagents and the like, and achieve the effects of cheap reaction reagents, short reaction route and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
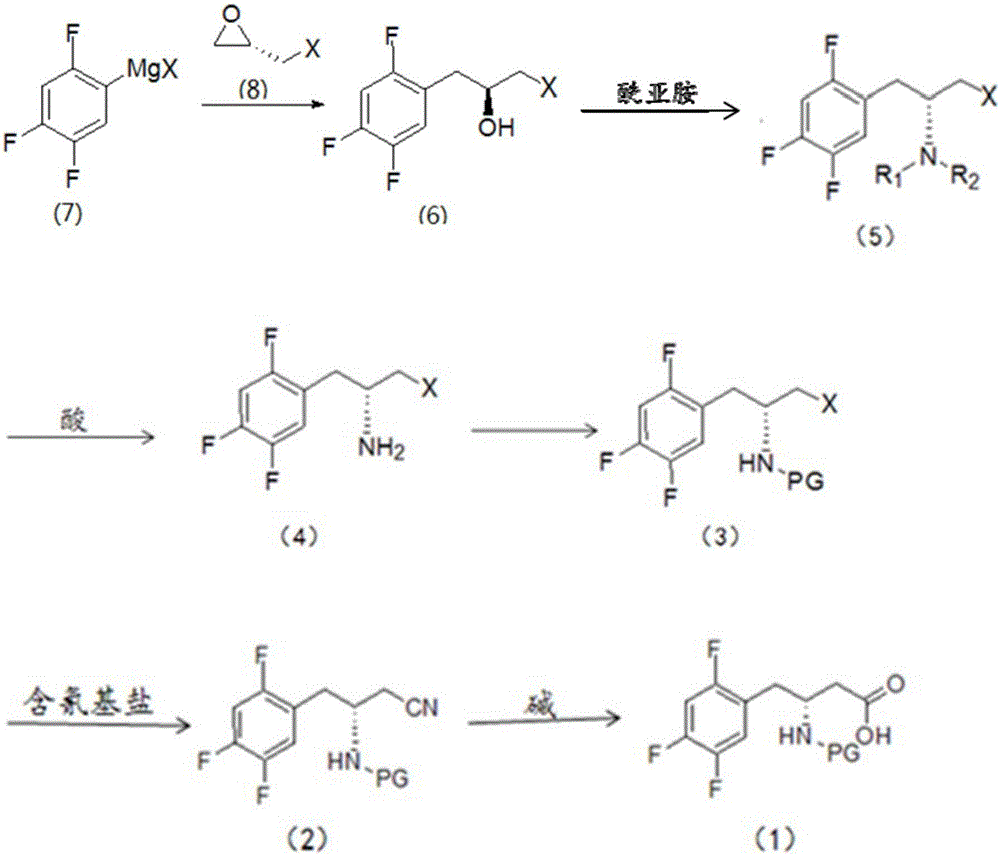
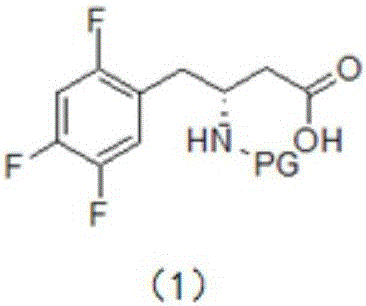
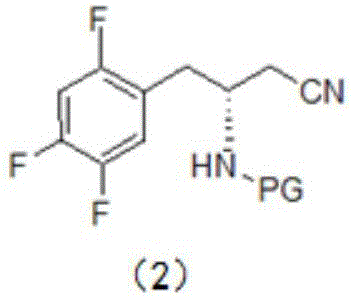
[0023] This embodiment provides a synthesis method for an intermediate of sitagliptin, the intermediate being a compound of formula (1), which includes: reacting a compound of formula (3) with a cyano-containing salt to obtain a compound of formula (2), and then hydrolyzing it with alkali Compound of formula (2);
[0024] The synthetic route of formula (1) compound is:
[0025]
[0026] Wherein, X is Cl, Br or I; PG is a nitrogen protecting group.
[0027] In the present invention, the compound of formula (3) is used as a reaction reagent, and the halogen in the compound of formula (3) is changed into a cyano group through a substitution reaction between the compound of formula (3) and a cyano-containing salt, and then the cyano group is dissolved in the alkali Hydrolysis to obtain the compound of formula (1), the intermediate of sitagliptin.
[0028] The compound of formula (2) is hydrolyzed under alkaline conditions to obtain the compound of formula (1). In a preferred ...
Embodiment 1
[0055] This embodiment provides a method for synthesizing an intermediate of sitagliptin, and the intermediate is a compound of formula (1-a).
[0056] Its synthetic route is as follows:
[0057]
[0058] Preparation of compounds of formula (2-a):
[0059] Mix tert-butyl (R)-[1-(2,4,5-trifluorophenyl)-3-chloropropan-2-yl]carbamate (34.0 g, 100 mmol) with 150 ml of dimethylsulfoxide , and then sequentially added 11.8g of NaCN and 30ml of polyethylene glycol-400 30ml; subsequently, the reaction solution was heated to 75-85°C for 6-10 hours.
[0060] Post-reaction treatment: the reaction solution was cooled to room temperature, mixed with 450ml of dichloromethane and 900ml of water and stirred, the organic layer was separated, washed with 100ml of water, dried with anhydrous sodium sulfate, and then concentrated under reduced pressure to remove the solvent to obtain the residue things. Add 100ml of isopropyl ether to the residue, stir the residue at room temperature, filter, ...
Embodiment 2
[0069] This embodiment provides a method for synthesizing an intermediate of sitagliptin, and the intermediate is a compound of formula (1-a).
[0070] Its synthetic route is as follows:
[0071]
[0072] Preparation of compounds of formula (4-a):
[0073] (R)-1-[1-(2,4,5-trifluorophenyl)-3-chloroprop-2-yl]pyrrolidine-2,5-dione (32.2g, 100mmol and 100g of water After mixing and dissolving, the temperature of the reaction solution was raised to 70-80° C., and then 10 g of 36% hydrochloric acid was slowly added dropwise. After the dropping, the temperature of the reaction solution was raised to 100° C. for reflux reaction for 14-16 hours.
[0074] Post-reaction treatment: the reaction solution was cooled to room temperature and stirred to precipitate a solid, which was filtered and dried in vacuum at 50-60°C to obtain 23.8 g of the compound of formula (4-a), with a yield of 86%.
[0075] Preparation of compounds of formula (3-a):
[0076] Mix (R)-1-chloromethyl-2-(2,4,5-tr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com