Preparation method of bilastine
A bilastine and compound technology, applied in the preparation field of bilastine, can solve the problems of complicated operation, harsh operation conditions, expensive raw materials and the like, and achieve the effects of simple preparation process, low overall cost and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
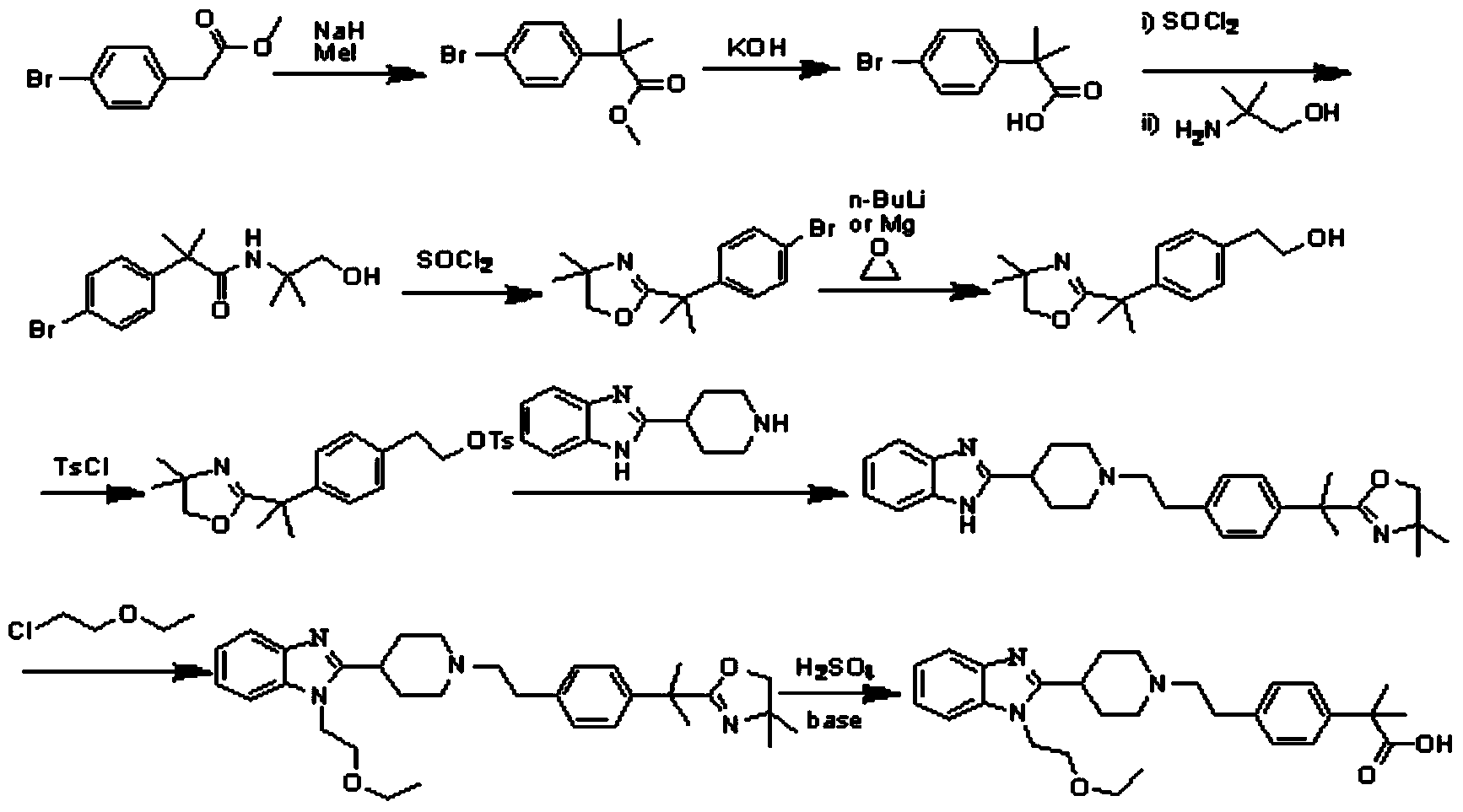
[0040] The present invention provides a method for preparing bilastine, including:
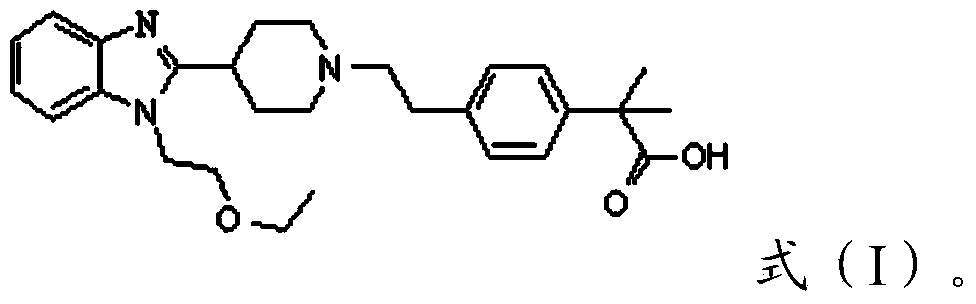
[0041] 1) Convert the compound with the structure of formula (II) into the compound with the structure of formula (III),
[0042]
[0043] Wherein, R is a C1-C4 alkyl group;
[0044] 2) Hydrolyze the compound having the structure of formula (III) to obtain bilastine.
[0045] According to the present invention, the compound with the structure of formula (II) is converted into the compound with the structure of formula (III) in the present invention. Specifically, the present invention preferably combines the compound with the structure of formula (II) and the methylating reagent under alkaline conditions. The following reaction yields a compound with the structure of formula (III), the methylating reagent is preferably dimethyl sulfate or methyl iodide; the base of the reaction is preferably sodium alkoxide, potassium tert-butoxide, sodium hydride, LiHMDS or two Lithium isopropylamide, more preferabl...
Embodiment 1
[0065] Preparation of methyl 2-[4-(2-hydroxyethylphenyl)]acetate (compound of structure (VI))
[0066] At 0°C, in a nitrogen atmosphere, to a tetrahydrofuran (260 mL) solution in which 20.0 g of a compound of formula (VII) (wherein R is a methyl) dissolved in 30 minutes, 100 mL of 0.1N borane tetrahydrofuran solution was added dropwise. After finishing, warm to room temperature and stir for 2h, quench with 130mL 2N hydrochloric acid aqueous solution, concentrate under reduced pressure, add 500mL dichloromethane and 250mL 2N hydrochloric acid aqueous solution, separate the liquids, extract the aqueous phase with 250mL*2 dichloromethane, combine the organic phases. Water was dried over magnesium sulfate, filtered, and concentrated under reduced pressure to obtain 16.2 g of colorless oily methyl 2-[4-(2-hydroxyethylphenyl)]acetate with a yield of 87.2.
[0067] The methyl 2-[4-(2-hydroxyethylphenyl)]acetate prepared in Example 1 was detected by high performance liquid chromatography, ...
Embodiment 2
[0070] Preparation of methyl 2-[4-(2-p-toluenesulfonyloxyethylphenyl)]acetate (compound of structure (IV))
[0071] At room temperature, 135.0mL of triethylamine, 63.1g of the compound prepared in Example 1, 67.9g of p-toluenesulfonyl chloride, and 600mL of dry dichloromethane solution were added to the reaction flask and stirred for 2 hours. After the reaction, 300mL*3 Washed with purified water, dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain 97.9 g of yellow oily 2-[4-(2-p-toluenesulfonyloxyethylphenyl)]acetate methyl ester with a yield of 80.2 %.
[0072] The methyl 2-[4-(2-p-toluenesulfonyloxyethylphenyl)]acetate prepared in Example 2 was detected by high performance liquid chromatography, and the result showed that its purity was 97.4%;
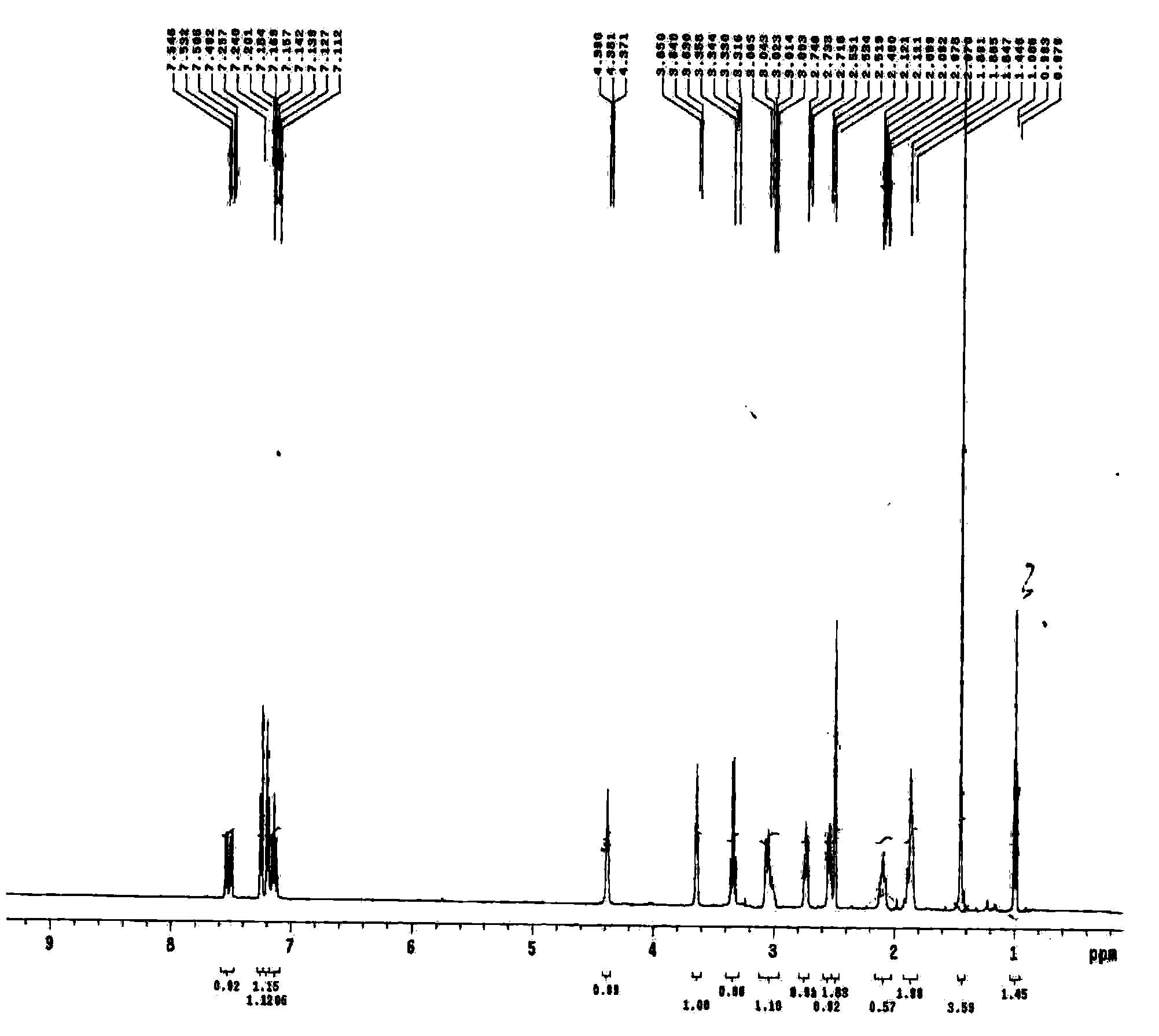
[0073] The methyl 2-[4-(2-p-toluenesulfonyloxyethylphenyl)]acetate prepared in Example 2 was detected by nuclear magnetism, and the results showed that 1 HNMR(CDCl 3 , 500MHz, TMS,)δ: 2.43(S,3H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com