Stable cefeclor dispersible tablet and preparation method thereof
A technology for cefaclor and dispersible tablets, which is applied in the field of stable cefaclor dispersible tablets and its preparation, can solve the problems of content drop, unstable light, high temperature, high humidity, reduced drug bioavailability, etc. Faster time, good press formability, small difference in tablet weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
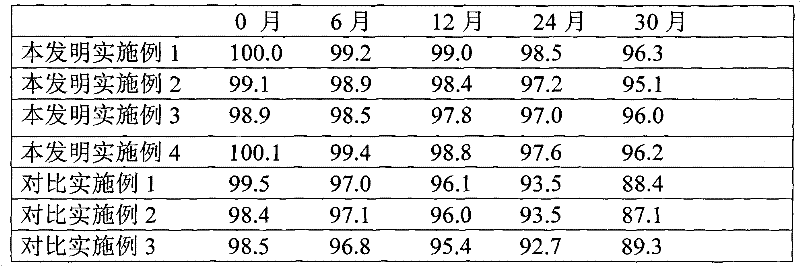
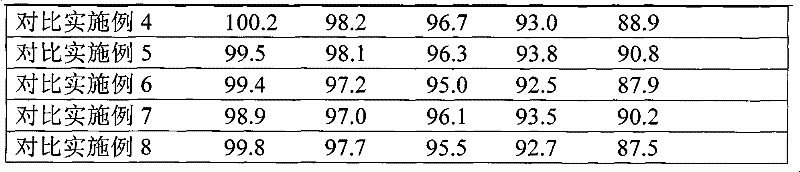
Embodiment 1
[0037] Embodiment 1 cefaclor dispersible tablet
[0038] Raw material name dosage
[0039] Cefaclor 25g
[0040] Microcrystalline Cellulose 11.5g
[0041] Hypromellose 7g
[0042] 2% hypromellose 40% ethanol solution appropriate amount
[0043] Pregelatinized starch 5g
[0044] Crospovidone 2.8g
[0045] Silica 1.2g
[0046] Magnesium Lauryl Sulfate 1g
[0047] Flavor 0.12g
[0048] Makes 100 pieces
[0049] Preparation:
[0050] 1) Handling of raw and auxiliary materials
[0051] Cefaclor is crushed through a 100-mesh sieve, microcrystalline cellulose, hydroxypropyl cellulose, pregelatinized starch, crospovidone, silicon dioxide, magnesium lauryl sulfate, and essence are sieved separately, and set aside.
[0052] 2) Making glue
[0053] Calculate the feeding amount of hypromellose, ethanol and water in the mucilage according to the prescription. Weigh the hypromellose, first add ethanol and stir to disperse evenly, then add water and stir fully to dissolve comple...
Embodiment 2
[0068] Embodiment 2 Cefaclor Dispersible Tablets
[0069]Raw material name dosage
[0070] Cefaclor 25g
[0071] Microcrystalline Cellulose 11.5g
[0072] Hypromellose 5g
[0073] 2% hypromellose 40% ethanol solution appropriate amount
[0074] Pregelatinized starch 5g
[0075] Crospovidone 3g
[0076] Silica 1.2g
[0077] Magnesium Lauryl Sulfate 1.5g
[0078] Flavor 0.12g
[0079] Makes 100 pieces
[0080] Preparation method: prepare with reference to the method of Example 1
Embodiment 3
[0081] Embodiment 3 cefaclor dispersible tablet
[0082] Raw material name dosage
[0083] Cefaclor 25g
[0084] Microcrystalline Cellulose 11.5g
[0085] Hypromellose 5g
[0086] 2% hypromellose 40% ethanol solution appropriate amount
[0087] Pregelatinized starch 5g
[0088] Crospovidone 4
[0089] Silica 1.2g
[0090] Magnesium Lauryl Sulfate 2g
[0091] Flavor 0.12g
[0092] Makes 100 pieces
[0093] Preparation method: refer to the method of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com