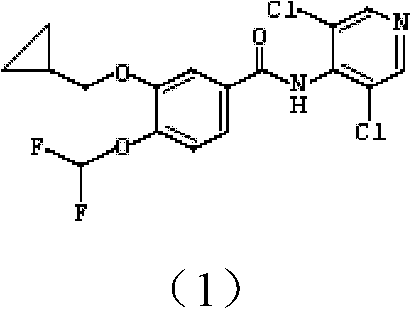
Pharmaceutical composition containing roflumilast
A technology of roflumilast and composition, which is applied in the field of pharmacy, can solve problems such as complicated operation technology, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weight ratio based on the tablet containing 0.5 mg roflumilast
[0024] 1. Roflumilast 0.76%
[0025] 2. Lactose 69.05%
[0026] 3. Corn starch 20.61%
[0027] 4. Crospovidone 7.49%
[0028] 5. Magnesium stearate 2.09%
[0029] Total 100%
[0030] Preparation Process:
[0031] Co-micronize the active ingredient roflumilast and part of lactose in the prescription, control the particle size of 90% volume or more particles in the range of 10-20um, pass all the excipients through 100 mesh respectively, and mix the micronized product with the remaining Measure lactose, corn starch, and crospovidone and mix them evenly in equal amounts, add an appropriate amount of 5% PVP K90, make soft materials, squeeze and sieve through a 18-mesh sieve to granulate, and dry the wet granules at a constant temperature at 50 degrees After 3 hours, the dry granules were sieved with a 24-mesh sieve, added with additional magnesium stearate, and mixed evenly.
[0032] A rotary tablet ...
Embodiment 2
[0035] Based on the weight of the tablet containing 0.125 mg roflumilast
[0036] 1. Roflumilast 0.11%
[0037] 2. Corn starch 47.01%
[0038] 3. Mannitol 42.11%
[0039] 4. Sodium carboxymethyl starch (internal addition) 5.00%
[0040] 5. Menthol 0.87%
[0041] 6. Sodium carboxymethyl starch (additional) 2.83%
[0042] 7. Magnesium stearate 2.06%
[0043] Total 100%
[0044] Preparation Process:
[0045] The active ingredient roflumilast in the prescription is micronized, and the particle size of 90% of the volume or more particles is controlled in the range of 10-30um,
[0046] All the excipients were passed through 100 meshes respectively, and the micronized roflumilast, corn starch, mannitol, sodium carboxymethyl starch, and menthol were mixed evenly in equal increments, and an appropriate amount of 5% hypromellose was added. Base cellulose, made of soft material, squeezed through a 18-mesh sieve and sieved to granulate. The wet granules were dried at a consta...
Embodiment 3
[0050] Based on the weight of the tablet containing 0.25 mg roflumilast
[0051] 1. Roflumilast 0.76%
[0052] 2. Corn starch 67.86%
[0053] 3. Sorbitol 22.14%
[0054] 4. Low-substituted hydroxypropyl cellulose 6.95%
[0055] 5. Magnesium stearate 2.29%g
[0056] Total 100%
[0057] Preparation Process:
[0058] The active ingredient roflumilast in the prescription is micronized, and the particle size of 90% of the volume or more particles is controlled within 50um, and all excipients are passed through 100 mesh respectively. Starch, sorbitol, and low-substituted hydroxypropyl cellulose are mixed uniformly by equal addition method, and an appropriate amount of 5% polyvinyl alcohol is added to make a soft material, which is extruded and sieved through a 18-mesh sieve to granulate, and the wet granules are placed at 50 degrees. Dry at constant temperature for 3 hours, sieve the dry granules through a 24-mesh sieve, add additional magnesium stearate, and mix well....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Sheet weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com