Novel synthetic method of high-purity florfenicol
A florfenicol and high-purity technology, applied in the new field of high-purity florfenicol synthesis, can solve the problem that the finished product cannot meet the high-standard florfenicol finished product, the high-purity florfenicol has high cost, and the single impurity exceeds the standard. Solvent and other problems, to achieve the effect of low cost, less impurities and shortened process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
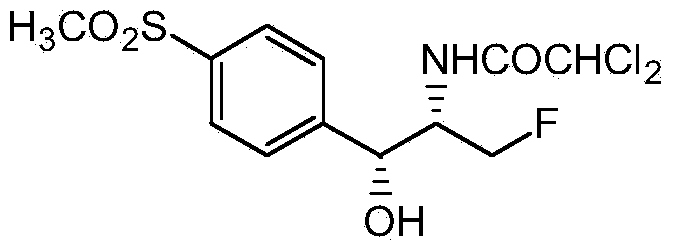
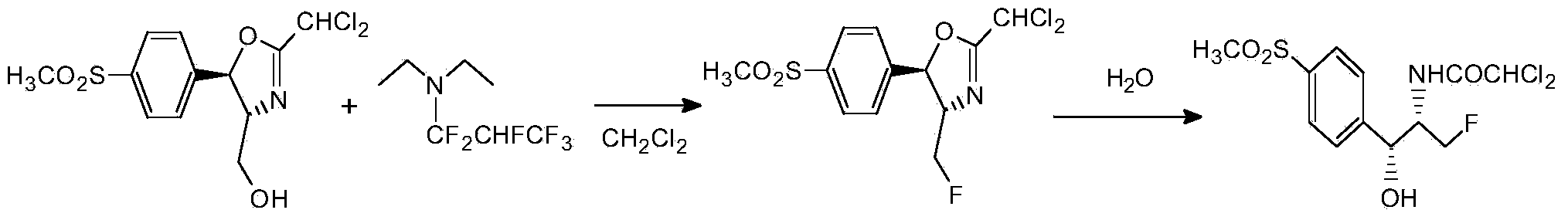
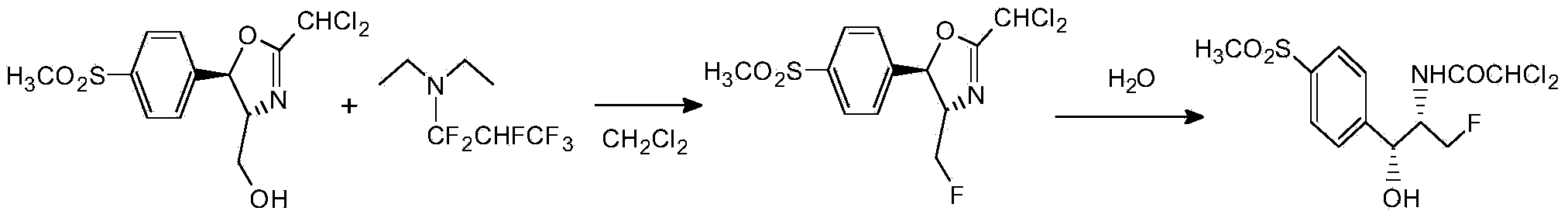
[0032] Dissolve 33 grams of diethylamine in 330 milliliters of dichloromethane, lower the temperature to -20 ° C, keep stirring, slowly pass in 75 grams of hexafluoropropylene, keep stirring for half an hour after passing through, transfer to a 1-liter high-pressure reactor, and add 100 Gram D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(methylsulfonyl)phenyl]-4-oxazolemethanol, the temperature was raised to 100°C, and the pressure in the kettle 0.55MPa, heat preservation reaction for 2 hours, take out to obtain a batch of fluorine substitution reaction liquid. Wash the fluorine substitution reaction solution with 500 ml of 10% aqueous sodium carbonate solution, stir for 0.5 hours, let stand to separate layers, wash the organic phase with 500 ml of water until the pH of the reaction solution is 7, distill part of the solvent at atmospheric pressure, and then cool down Crystallization, the crystals were filtered at 0°C to obtain white high-purity fluorinated product (4S,5R)-2-(di...
Embodiment 2
[0034] Dissolve 33 grams of diethylamine in 330 milliliters of dichloromethane, lower the temperature to -20 ° C, keep stirring, slowly pass in 75 grams of hexafluoropropylene, keep stirring for half an hour after passing through, transfer to a 1-liter high-pressure reactor, and add 100 Gram D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(methylsulfonyl)phenyl]-4-oxazolemethanol, the temperature was raised to 100°C, and the pressure in the kettle 0.55MPa, heat preservation reaction for 2 hours, take out to obtain a batch of fluorine substitution reaction liquid. The fluorine substitution reaction solution was first washed with 500 ml of 10% sodium acetate aqueous solution, stirred for 0.5 hours, allowed to stand for stratification, and the organic phase was washed with 500 ml of water until the pH of the reaction solution was 7. After distilling part of the solvent at atmospheric pressure, the temperature was lowered. Crystallization, the crystals were filtered at 0°C to obtain w...
Embodiment 3
[0036] Dissolve 33 grams of diethylamine in 330 milliliters of dichloromethane, lower the temperature to -20 ° C, keep stirring, slowly pass in 75 grams of hexafluoropropylene, keep stirring for half an hour after passing through, transfer to a 1-liter high-pressure reactor, and add 100 Gram D-threo-2-(dichloromethyl)-4,5-dihydro-5-[p-(methylsulfonyl)phenyl]-4-oxazolemethanol, the temperature was raised to 100°C, and the pressure in the kettle 0.55MPa, heat preservation reaction for 2 hours, take out to obtain a batch of fluorine substitution reaction liquid. The fluorine substitution reaction solution was first washed with 500 milliliters of 10% aqueous sodium bicarbonate solution, stirred for 0.5 hours, allowed to stand for stratification, and the organic phase was washed with 500 milliliters of water until the pH of the reaction solution was 7. After distilling part of the solvent under normal pressure, Cool down to crystallize, and filter the crystals at 0°C to obtain a wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com