Compound ammonium glycyrrhizinato S dispersed tablet and its preparing process
A technology of compound licorice and glycyrrhizic acid monoammonium salt, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of injection production process requirements, inconvenient use, and poor stability, and achieve Guaranteed bioavailability, easy portability, and reliable quality control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1), the original and auxiliary materials are crushed through a 100-mesh sieve;
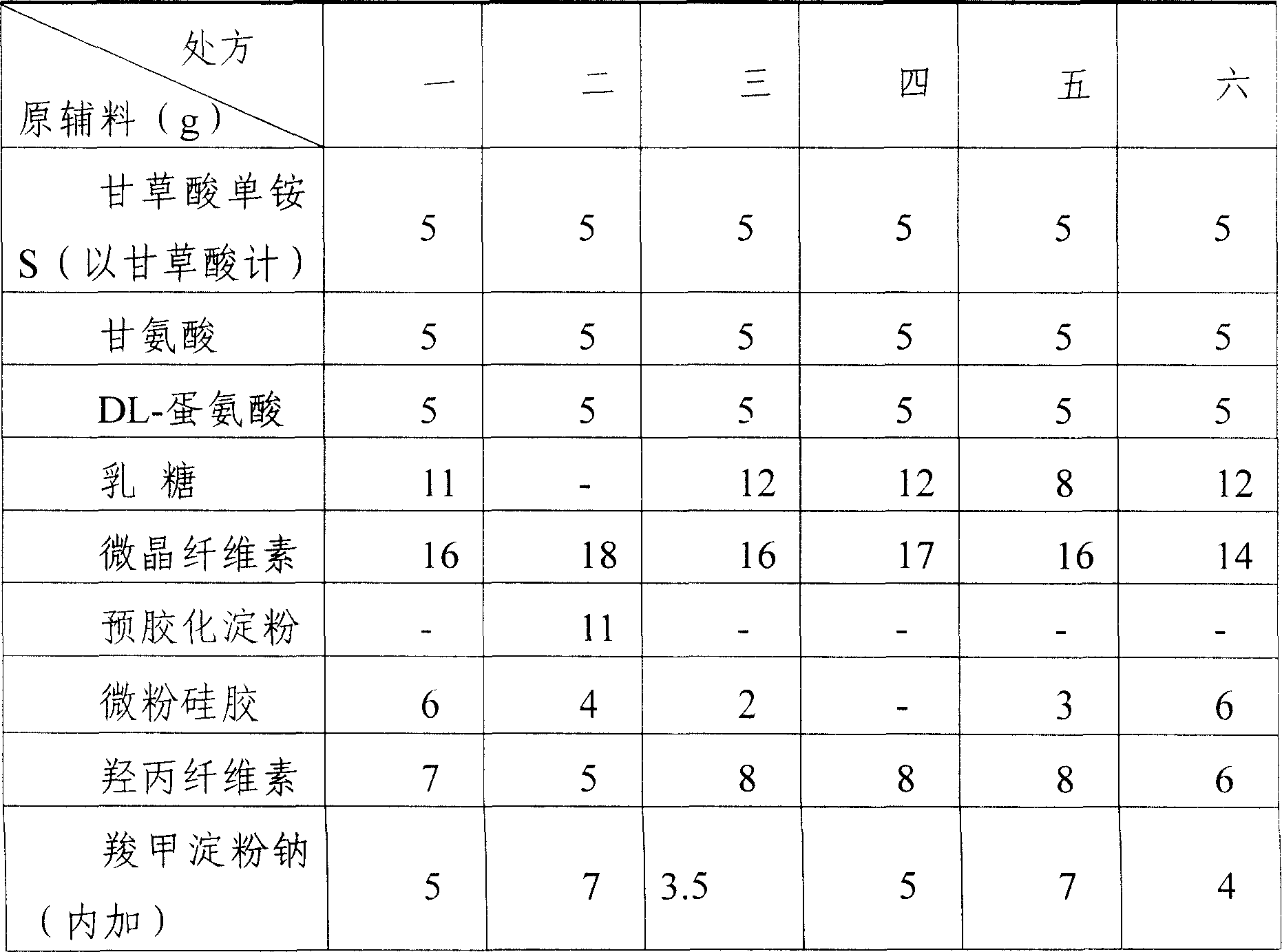
[0021] 2) Weigh and take 25g of monoammonium glycyrrhizinate S (calculated as glycyrrhizic acid), 25g of glycine, 25g of DL-methionine, 80g of microcrystalline cellulose, 40g of hydroxypropyl cellulose (low substitution), 40g of lactose, and micronized silica gel 15g, crospovidone 9g, sodium carboxymethyl starch 35g, mix homogeneously by the method of increasing in equal amounts, add 2% PVP ethanol (40%) solution, make soft material, soft material crosses 20 mesh sieves and granulates;
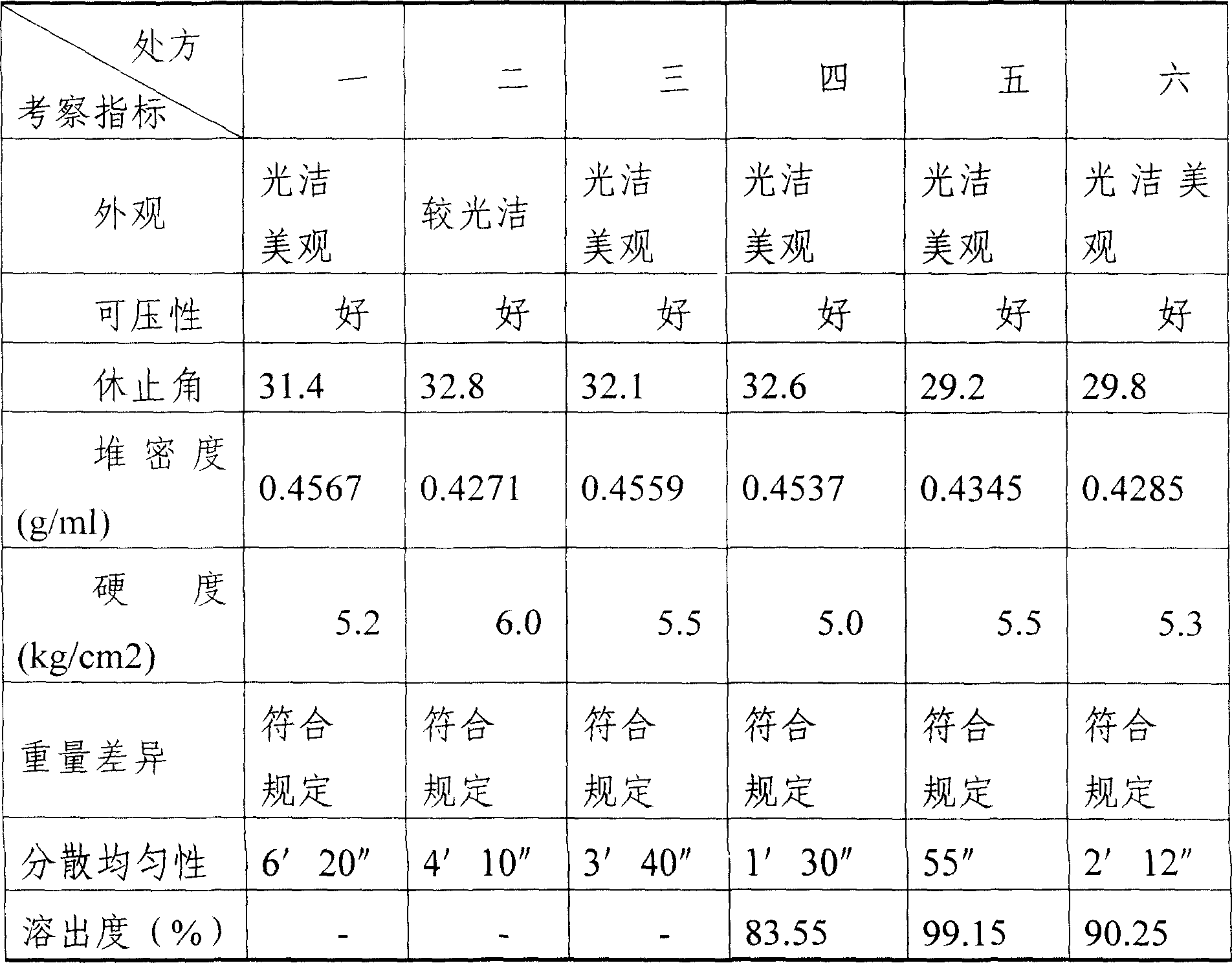
[0022] 3) Dry the wet granules at 60°C to 70°C for 3 to 4 hours, then sieve the granules with a 20-mesh sieve, add 0.62g of talc powder and 7g of crospovidone, mix evenly, and measure the content of the semi-finished product;
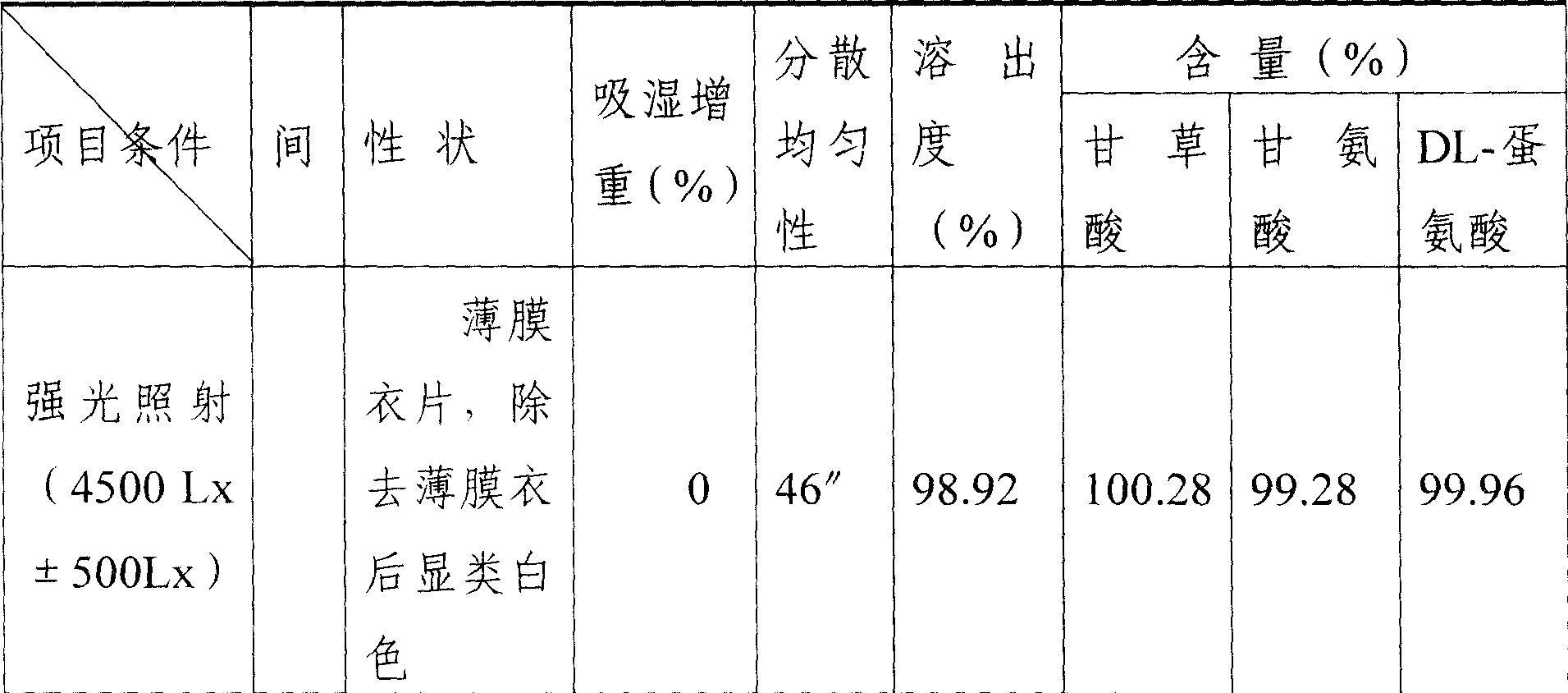
[0023] 4) After the content of the semi-finished product is qualified, use a circular chamfering die with a diameter of 11mm to press the tablet. The sheet shape is round, and the sh...
Embodiment 2
[0026] 1), the original and auxiliary materials are crushed through a 100-mesh sieve;
[0027] 2) Weigh 5g of monoammonium glycyrrhizinate S (calculated as glycyrrhizic acid), 30g of glycine, 5g of DL-methionine, 80g of microcrystalline cellulose, 40g of hydroxypropyl cellulose (low substitution), 40g of lactose, and micronized silica gel 15g, crospovidone 9g, sodium carboxymethyl starch 35g, mix homogeneously by the method of increasing in equal amounts, add 2% PVP ethanol (40%) solution, make soft material, soft material crosses 20 mesh sieves and granulates;
[0028] 3) Dry the wet granules at 60°C to 70°C for 3 to 4 hours, then sieve the granules with a 20-mesh sieve, add 0.62g of talc powder and 7g of crospovidone, mix evenly, and measure the content of the semi-finished product;
[0029] 4) After the content of the semi-finished product is qualified, use a circular chamfering die with a diameter of 11mm to press the tablet. The sheet shape is round, and the sheet weight ...
Embodiment 3
[0032] 1), the original and auxiliary materials are crushed through a 100-mesh sieve;
[0033] 2) Weigh and take 30g of monoammonium glycyrrhizinate S (calculated as glycyrrhizic acid), 5g of glycine, 30g of DL-methionine, 80g of microcrystalline cellulose, 40g of hydroxypropyl cellulose (low substitution), 40g of lactose, and micronized silica gel 15g, crospovidone 9g, sodium carboxymethyl starch 35g, mix homogeneously by the method of increasing in equal amounts, add 2% PVP ethanol (40%) solution, make soft material, soft material crosses 20 mesh sieves and granulates;
[0034] 3) Dry the wet granules at 60°C to 70°C for 3 to 4 hours, then sieve the granules with a 20-mesh sieve, add 0.62g of talc powder and 7g of crospovidone, mix evenly, and measure the content of the semi-finished product;
[0035] 4) After the content of the semi-finished product is qualified, use a circular chamfering die with a diameter of 11mm to press the tablet. The sheet shape is round, and the she...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com