Esomeprazole and preparation method of magnesium trihydrate of esomeprazole
A technology for esomeprazole and omeprazole sodium salt, which is applied in the field of drug synthesis, can solve the problems of low esomeprazole yield, low enantiomeric excess value, unsuitable for industrial production and the like, and achieves cost Low, short reaction time, easy refining effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
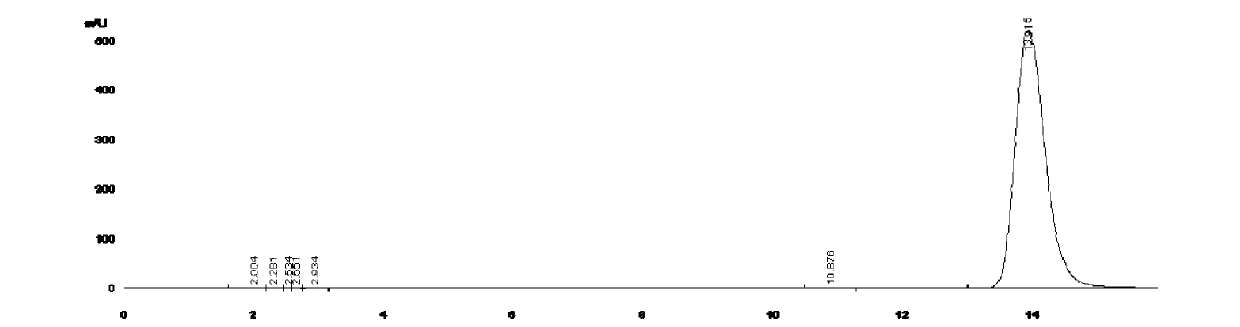
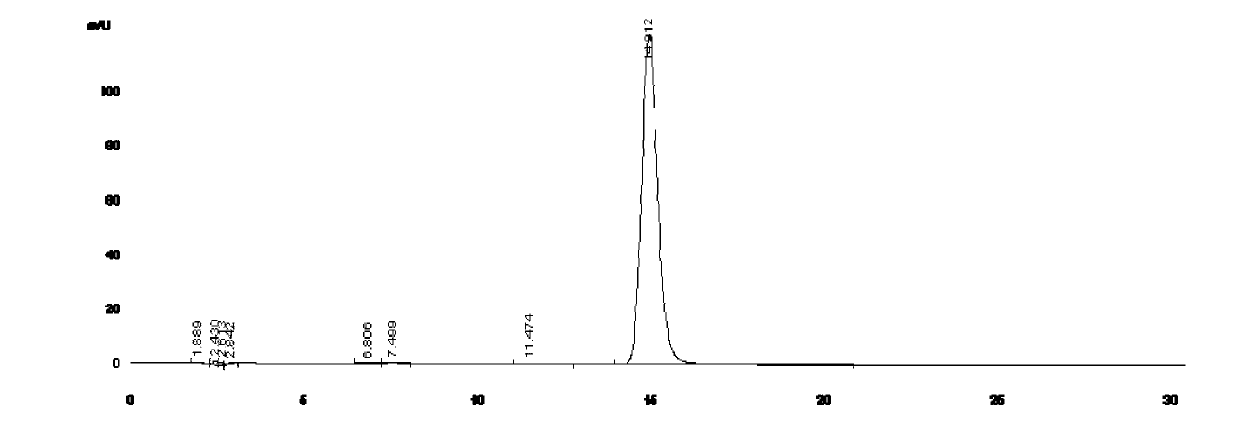
Image
Examples
preparation example Construction
[0038] One aspect of the present invention provides a kind of preparation method of esomeprazole, comprises the following steps:
[0039] (1) Carrying out acid-base neutralization reaction of racemic omeprazole and inorganic base in alcohol solution to obtain omeprazole sodium salt;
[0040] (2) Dissolving omeprazole sodium salt, organometallic complexing agent, chelating agent, and organic base in an organic solvent for complexation reaction to obtain esomeprazole complex;
[0041] (3) Condensing the esomeprazole complex with S-mandelic acid to obtain the esomeprazole mandelate complex;
[0042] (4) dissolving the esomeprazole mandelate complex in an acetone solution and filtering to obtain the S-omeprazole-S-mandelate complex;
[0043] (5) Suspending the S-omeprazole-S-mandelate complex in the first solvent to obtain a suspension, adjusting the pH of the suspension to 8~10 to obtain esomeprazole, the first solvent includes 30~32v / v% alkaline aqueous solution and 68~70v / v% ...
Embodiment 1
[0067] Preparation of esomeprazole:
[0068] (1) Weigh 5L of methanol and 8L of isopropanol into a 20L reactor, weigh 348.0g of sodium hydroxide and put it into the reactor, adjust the temperature in the reactor to 20°C, and stir mechanically for 0.5h to obtain solution 1. Suction filter the solution with diatomaceous earth to obtain the filtrate, take 10 L of the filtrate into the reaction kettle, add 2 kg of racemic omeprazole, stir mechanically for 2 hours, and spin dry under reduced pressure at 45°C to obtain a white solid. Add 4L of ethyl acetate to the reaction kettle containing the obtained white solid, stir for 1 hour, filter with suction, wash the filter cake with 1.6L of ethyl acetate, and dry the filter cake at 40°C for 8 hours to obtain 2.2kg with a water content of 1.5%. omeprazole sodium white solid, 2.2kg of omeprazole sodium is ground into powder, added to 2.5L cyclohexane and 193ml deionized water, and the mixed solution formed continues to stir for 2h and the...
Embodiment 2
[0078] Preparation of esomeprazole:
[0079](1) Weigh 5L of methanol and 8L of isopropanol into a 20L reactor, weigh 348.0g of sodium hydroxide and put it into the reactor, adjust the temperature of the reactor to 25°C, and stir mechanically for 0.5h to obtain solution 1. Suction filter the solution with diatomaceous earth to obtain the filtrate, take 10 L of the filtrate into the reaction kettle, add 2 kg of racemic omeprazole, stir mechanically for 2 hours, and spin dry under reduced pressure at 45°C to obtain a white solid. Add 4L of ethyl acetate to the reaction kettle containing the obtained white solid and stir for 1h, filter with suction, wash the filter cake with 1.6L of ethyl acetate, and dry the filter cake at 40°C for 8h to obtain 2.2kg of omeprazole Sodium white solid, 2.2kg of omeprazole sodium is ground into powder, added to 2.5L of cyclohexane and 193ml of deionized water, and the resulting mixed solution is continuously stirred for 2 hours, then suction filtere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com