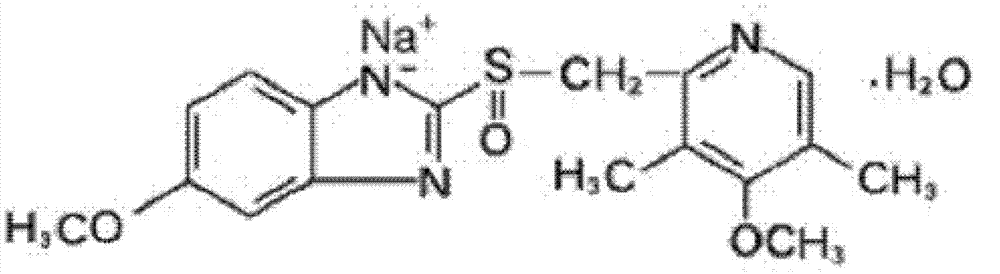
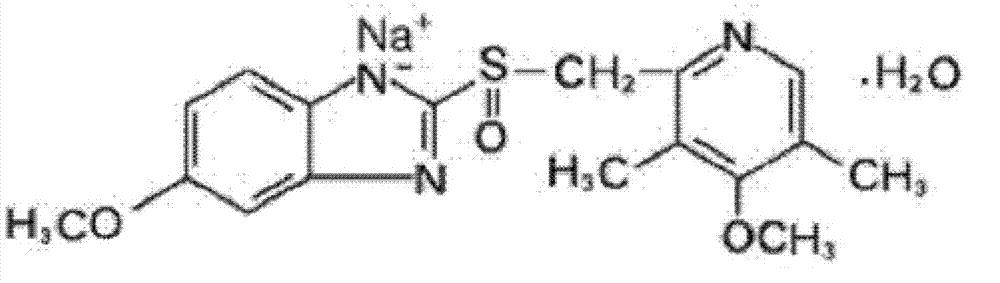
Omeprazole sodium freeze-dried powder injection for injection
A technology of freeze-dried powder injection and omeprazole sodium, which is applied in the field of medicine and can solve the problems of instability of freeze-dried powder and increasing the moisture content of freeze-dried powder.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0092] Preparation example 1, prepare the powder injection comprising omeprazole sodium
[0093] formula:
[0094] Omeprazole Sodium
40mg,
5 mg,
EDTA-2Na
1.5 mg,
pH regulator
to pH10.5,
Water for Injection
Appropriate amount, add to 1ml.
[0095] Preparation:
[0096] (1) Take the main drug and auxiliary materials of the prescription amount, place them in a stainless steel bucket, add about 80% of the prescription amount of water for injection to dissolve each component, and then add 0.1% (w / v) of activated carbon according to the volume of the solution, Stir for 30 minutes, filter and decarburize, and add water for injection to nearly the full amount of the prescription.
[0097] (2) The filtrate is sampled, and the pH value is measured, and if necessary, it is adjusted to a specified value with a pH regulator (this specified value is the value of the measured pH value of the freeze-dried gained d...
Embodiment 1
[0108]
[0109] Stability study example: Measure the chemical stability of the sample prepared in the above embodiment 1 part according to the chemical stability investigation method of this paper, after placing 4 months at 45 ° C, especially measure their residual content (%), total special impurity increment (%) ) and total miscellaneous increment (%), the results are shown in Table 1 below.
[0110] Table 1
[0111] sample
Residual content (%)
Total miscellaneous increment (%)
Total Miscellaneous Increment (%)
Ex100
96.6
340
435
Ex101
97.1
270
311
Ex102
98.3
155
244
Ex103
99.1
42
78
Ex104
99.2
33
69
Ex1
99.4
35
73
Ex106
99.2
34
83
[0112] Ex107
99.3
45
78
Ex108
98.4
52
93
Ex109
98.3
58
112
Ex110
98.5
63
144
Ex115
98.0
...
preparation example 2
[0119] Preparation example 2, powder injection of the present invention
[0120] The prescription and production method refer to Preparation Example 1, the only difference is that the dosage of EDTA-2Na is changed to 1mg or 2mg, and two batches of powder injections are obtained, and the numbers are respectively recorded as Ex21 and Ex22.
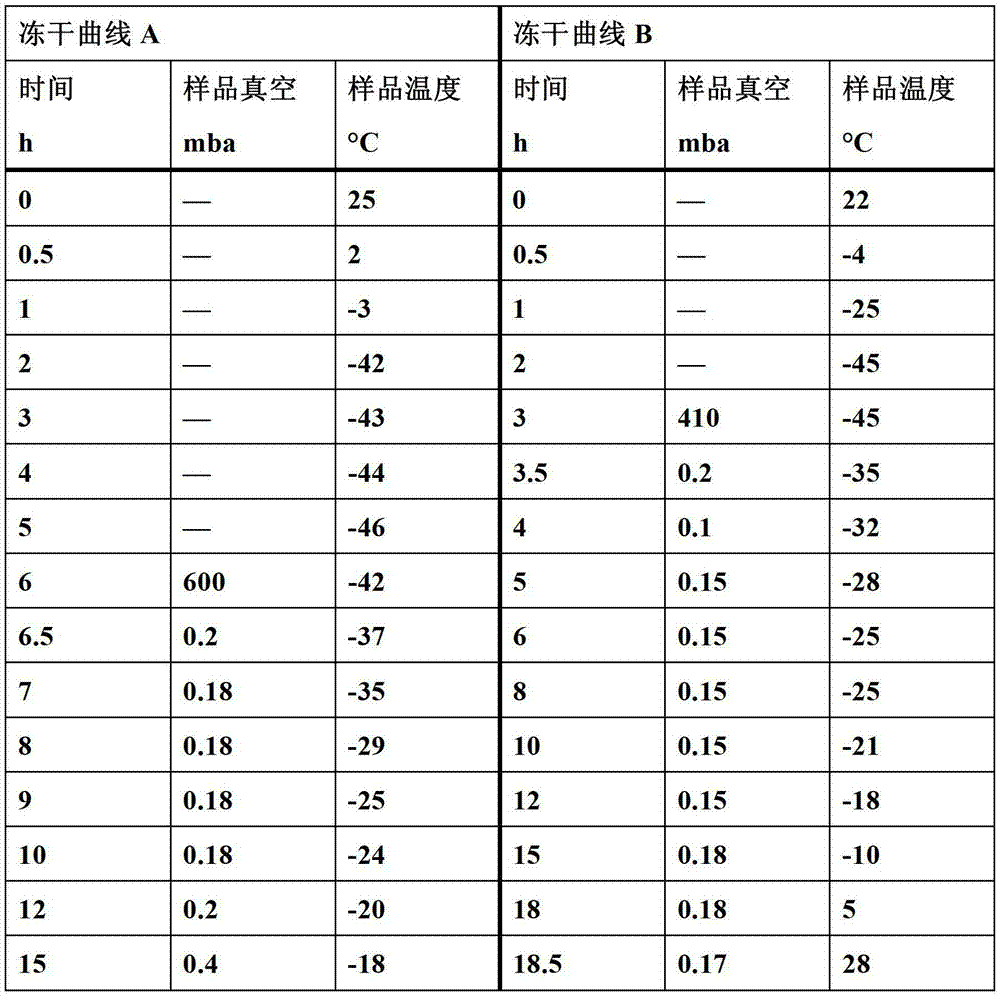
[0121] The prescription and preparation method refer to Preparation Example 1, the only difference is that the pH value is controlled to 10.0 or 11.0, and the freeze-drying curve B described in this article is used for freeze-drying to obtain two batches of powder injections, and the numbers are respectively recorded as Ex23 , Ex24.
[0122] The prescription and preparation method refer to Preparation Example 1, the only difference is that the amount of water for injection is adjusted so that the solid content of the filtrate obtained in step (3) is 4%, 7%, and 10%, and three batches of powder injections are obtained. They are recorded as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com